Your Acid base neutralization reaction example images are available in this site. Acid base neutralization reaction example are a topic that is being searched for and liked by netizens today. You can Get the Acid base neutralization reaction example files here. Download all royalty-free photos.
If you’re searching for acid base neutralization reaction example pictures information related to the acid base neutralization reaction example keyword, you have visit the ideal blog. Our website frequently provides you with suggestions for refferencing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and images that fit your interests.
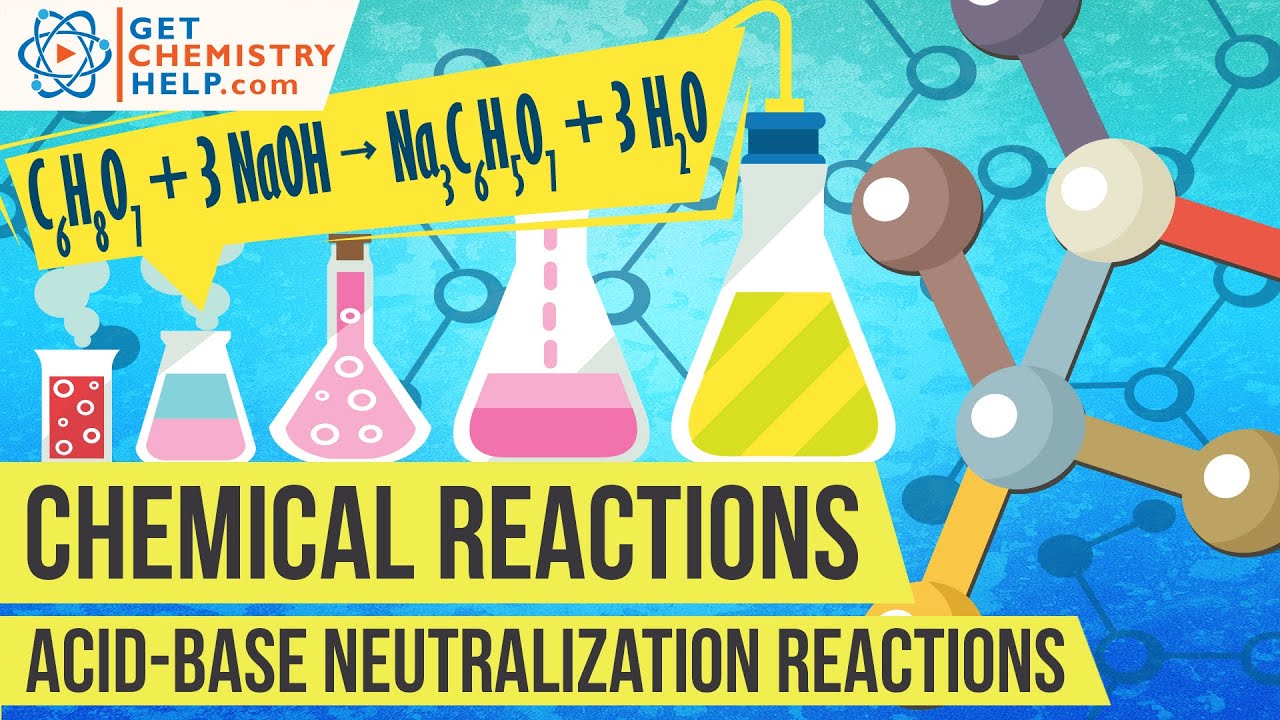
Acid Base Neutralization Reaction Example. BrønstedLowry Acid The species molecule or ion that donates a proton H to another species in a protontransfer. What is an example of an acid-base neutralization reaction. Then we will balance the equation. The acid-base neutralization reaction is a reaction that occurs when an equal amounts of acids and bases of the same strength is used to form a neutral solution.
 Pin On Teaching Chemistry From pinterest.com
Pin On Teaching Chemistry From pinterest.com
The neutralized solution usually has a ph seven value but the pH value can. Hence the name neutralization. A neutralisation reaction is generally an acid-base neutralization reaction. A neutralization reaction is when an acid and a base react to form water and salt and involves the combination of hydrogen ions and hydroxyl ions to generate water. At the end of the reaction the solution overall becomes neutral. Acid-base neutralization reaction is an example of one type of chemical reactions.
This reaction is called neutralization and the equation is.
Acid Base rightarrow Salt Water. Acid base water salt. Arrhenius Base A substance that produces hydroxide ions OH when dissolved in water. The neutralized solution usually has a ph seven value but the pH value can. Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater. Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt.
 Source: pinterest.com
Source: pinterest.com
A salt is the product of an acid-base reaction and is a much broader term then common table salt as shown in the first reaction. Moreover many of the substances we encounter in our homes the supermarket and the pharmacy are acids or bases. Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater. In the reaction H and OH-combine to form HOH or H 2 O or water molecules. 132 Acid-base reactions ESBQY The reaction between an acid and a base is known as a neutralisation reaction.
 Source: pinterest.com
Source: pinterest.com
Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater. Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater. H 2 SO 4 aq 2 NaOHaq Æ Na 2 SO 4 aq 2 H 2 Ol Recall that molarity M is expressed as. Neutralization Reactions acid-base Acids Bases Arrhenius Acid A substance that produces hydrogen ions H when dissolved in water. Moreover many of the substances we encounter in our homes the supermarket and the pharmacy are acids or bases.
 Source: pinterest.com
Source: pinterest.com
In acid-base neutralization reaction an acid reacts with base to produce salt and water. First we will write the chemical equation with the formulas of the reactants and the expected products. A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. For example K 2 CO 3 is formed due to the acid-base reaction of potassium hydroxide strong base and H 2 CO 3 weak acid. Neutralization reactions actually take place between the acidic proton from the acid and the hydroxyl group of the base to generate water H and OH-react to form H 2 O.
 Source: in.pinterest.com
Source: in.pinterest.com
Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. Moreover many of the substances we encounter in our homes the supermarket and the pharmacy are acids or bases. We define neutralization as the chemical reaction between an acid and a base. Acid Base rightarrow Salt Water. The chemical process by which an equivalent amount of acid reacts with an equivalent amount of alkali results in the complete disappearance of acid and alkali properties producing salt and water that chemical process is called acid-base neutralization reaction.
 Source: pinterest.com
Source: pinterest.com
Acid base salt water. Write the neutralization reactions between each acid and base. For example K 2 CO 3 is formed due to the acid-base reaction of potassium hydroxide strong base and H 2 CO 3 weak acid. Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater. The acid and base are reacted away and neither remains.
 Source: in.pinterest.com
Source: in.pinterest.com
A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. We define neutralization as the chemical reaction between an acid and a base. A salt is the product of an acid-base reaction and is a much broader term then common table salt as shown in the first reaction. Acid-Base Neutralization Reactions. The reaction between an acid and a base is called an acid-base reaction or a neutralization reaction.
 Source: pinterest.com
Source: pinterest.com
Neutralization Reactions acid-base Acids Bases Arrhenius Acid A substance that produces hydrogen ions H when dissolved in water. A neutralisation reaction is generally an acid-base neutralization reaction. Then we will balance the equation. HNO 3 aq and BaOH 2 aq H 3 PO 4 aq and CaOH 2aq Solution. Example Calculation In an acid-base titration 5000 mL of 05000 M sodium hydroxide is needed to neutralize 3226 mL of sulfuric acid.
 Source: pinterest.com
Source: pinterest.com
BrønstedLowry Acid The species molecule or ion that donates a proton H to another species in a protontransfer. We will look at a few examples of acid-base reactions. Milk of magnesia which is a base is given as antacid in the case of indigestion to neutralize the more acid produced in the stomach. Example Calculation In an acid-base titration 5000 mL of 05000 M sodium hydroxide is needed to neutralize 3226 mL of sulfuric acid. Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater.
 Source: pinterest.com
Source: pinterest.com
A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. H 2 CO 3 2KOH K 2 CO 3 2H 2 O When a weak acid and weak base react with each other complete neutralization does not occur due to incomplete ionization of the acid and base. In this section we tackle the acid-base neutralization reactions which are essential in both biochemistry and industrial chemistry. Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. The cation form the base Na ion and the anion from the acid Cl ion reacts together to form the salt.
 Source: pinterest.com
Source: pinterest.com
Acid-Base Neutralization Reactions. For example if we mix hydrochloric acid with sodium hydroxide they will react and produce sodium chloride and water. Moreover many of the substances we encounter in our homes the supermarket and the pharmacy are acids or bases. What is the molarity of the aqueous sulfuric acid solution. For example K 2 CO 3 is formed due to the acid-base reaction of potassium hydroxide strong base and H 2 CO 3 weak acid.
 Source: pinterest.com
Source: pinterest.com
The most common example of neutralization is the reaction between hydrochloric acid an acid and sodium hydroxide a base. Examples of neutralization reaction July 18 2019 247 pm A neutralization is a chemical reaction in which the reactants are an acid and a base and the products are a salt and water. Example Calculation In an acid-base titration 5000 mL of 05000 M sodium hydroxide is needed to neutralize 3226 mL of sulfuric acid. Although acids and bases have their own unique chemistries the acid and base cancel each others chemistry to produce a rather innocuous substancewater. First we will write the chemical equation with the formulas of the reactants and the expected products.
 Source: pinterest.com
Source: pinterest.com
Often when an acid and base react a salt and water will be formed. HNO 3 aq and BaOH 2 aq H 3 PO 4 aq and CaOH 2aq Solution. Examples of neutralization reaction July 18 2019 247 pm A neutralization is a chemical reaction in which the reactants are an acid and a base and the products are a salt and water. In a neutralization reaction there is a combination of H ions and OH ions which form water. Neutralization Reactions acid-base Acids Bases Arrhenius Acid A substance that produces hydrogen ions H when dissolved in water.
 Source: pinterest.com
Source: pinterest.com
A salt is the product of an acid-base reaction and is a much broader term then common table salt as shown in the first reaction. Milk of magnesia which is a base is given as antacid in the case of indigestion to neutralize the more acid produced in the stomach. The neutralization reaction is also known as the acid base neutralization reaction or just the acid base reaction. Acid-Base Neutralization Reactions. At the end of the reaction the solution overall becomes neutral.
 Source: pinterest.com
Source: pinterest.com
For example if we mix hydrochloric acid with sodium hydroxide they will react and produce sodium chloride and water. In the reaction H and OH-combine to form HOH or H 2 O or water molecules. At the end of the reaction the solution overall becomes neutral. In acid-base neutralization reaction an acid reacts with base to produce salt and water. A neutralisation reaction is generally an acid-base neutralization reaction.
 Source: pinterest.com
Source: pinterest.com
Arrhenius Base A substance that produces hydroxide ions OH when dissolved in water. The neutralized solution usually has a ph seven value but the pH value can. Learn about the net ionic equation review examples of. Hence the name neutralization. Then we will balance the equation.
 Source: pinterest.com
Source: pinterest.com
There are many examples where this is useful. HNO 3 aq and BaOH 2 aq H 3 PO 4 aq and CaOH 2aq Solution. Write the neutralization reaction. H 2 CO 3 2KOH K 2 CO 3 2H 2 O When a weak acid and weak base react with each other complete neutralization does not occur due to incomplete ionization of the acid and base. What is an example of an acid-base neutralization reaction.
 Source: pinterest.com
Source: pinterest.com
Neutralization is a chemical reaction through which water and salt are formed as the result of a strong acid and strong base coming together. Examples of neutralization reaction July 18 2019 247 pm A neutralization is a chemical reaction in which the reactants are an acid and a base and the products are a salt and water. Hence the name neutralization. In the reaction H and OH-combine to form HOH or H 2 O or water molecules. For example K 2 CO 3 is formed due to the acid-base reaction of potassium hydroxide strong base and H 2 CO 3 weak acid.
 Source: pinterest.com
Source: pinterest.com
A neutralization is a type of double replacement reaction. H 2 CO 3 2KOH K 2 CO 3 2H 2 O When a weak acid and weak base react with each other complete neutralization does not occur due to incomplete ionization of the acid and base. The acid-base neutralization reaction is a reaction that occurs when an equal amounts of acids and bases of the same strength is used to form a neutral solution. In the reaction H and OH-combine to form HOH or H 2 O or water molecules. The reaction yields salt and water molecules.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title acid base neutralization reaction example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






