Your Alpha decay equation example images are ready. Alpha decay equation example are a topic that is being searched for and liked by netizens today. You can Get the Alpha decay equation example files here. Download all royalty-free photos and vectors.
If you’re looking for alpha decay equation example pictures information connected with to the alpha decay equation example keyword, you have visit the ideal site. Our website always provides you with hints for downloading the maximum quality video and image content, please kindly hunt and find more enlightening video articles and graphics that match your interests.
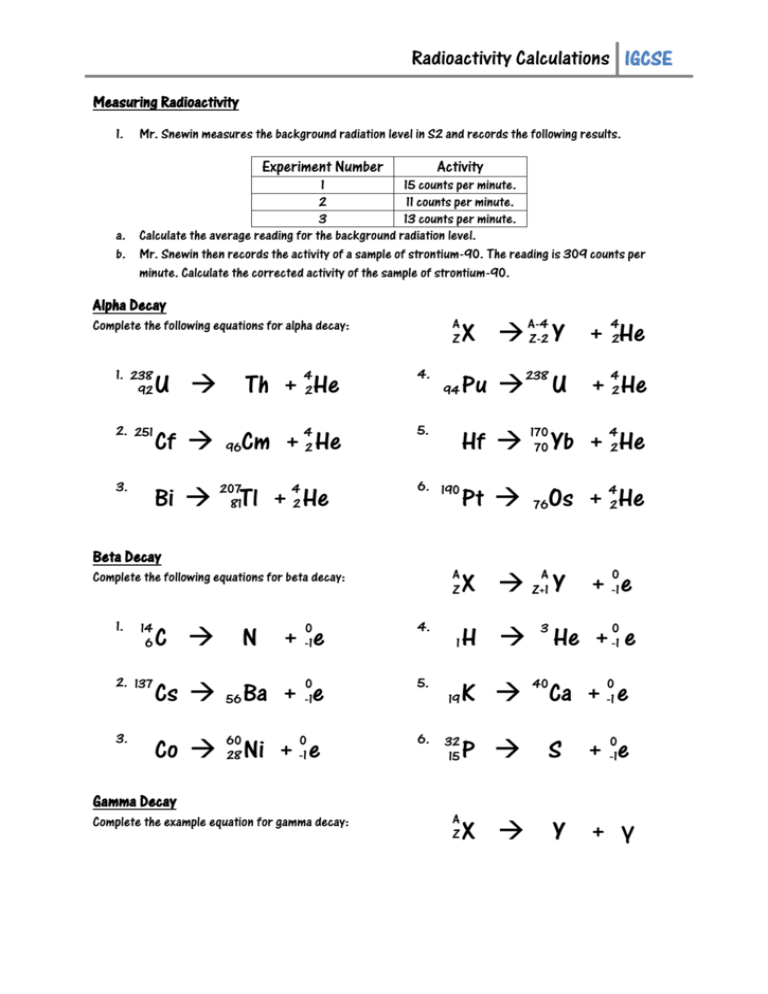
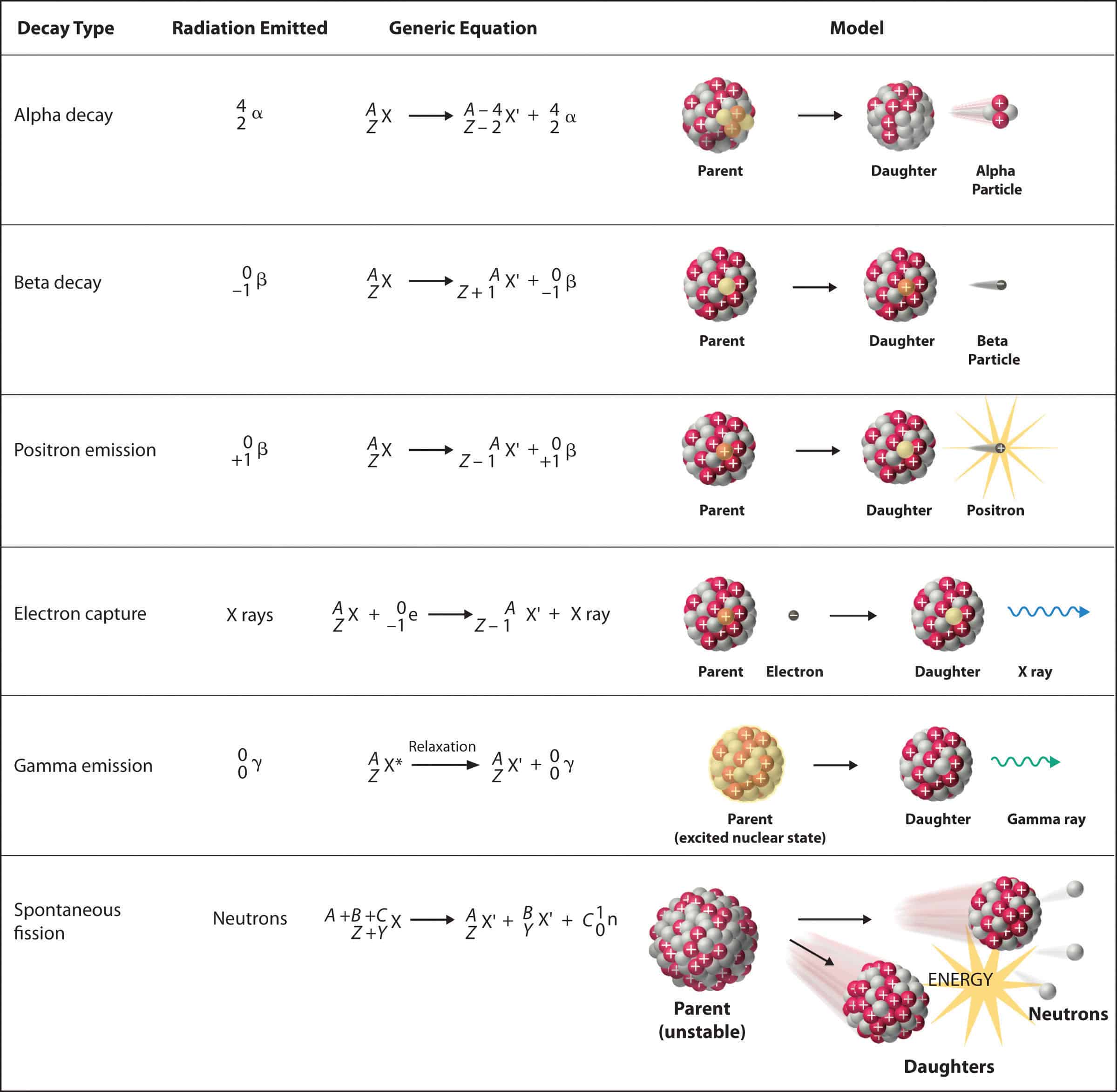
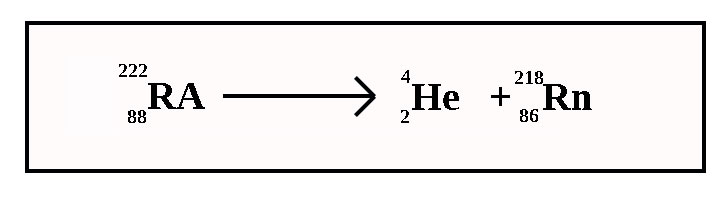
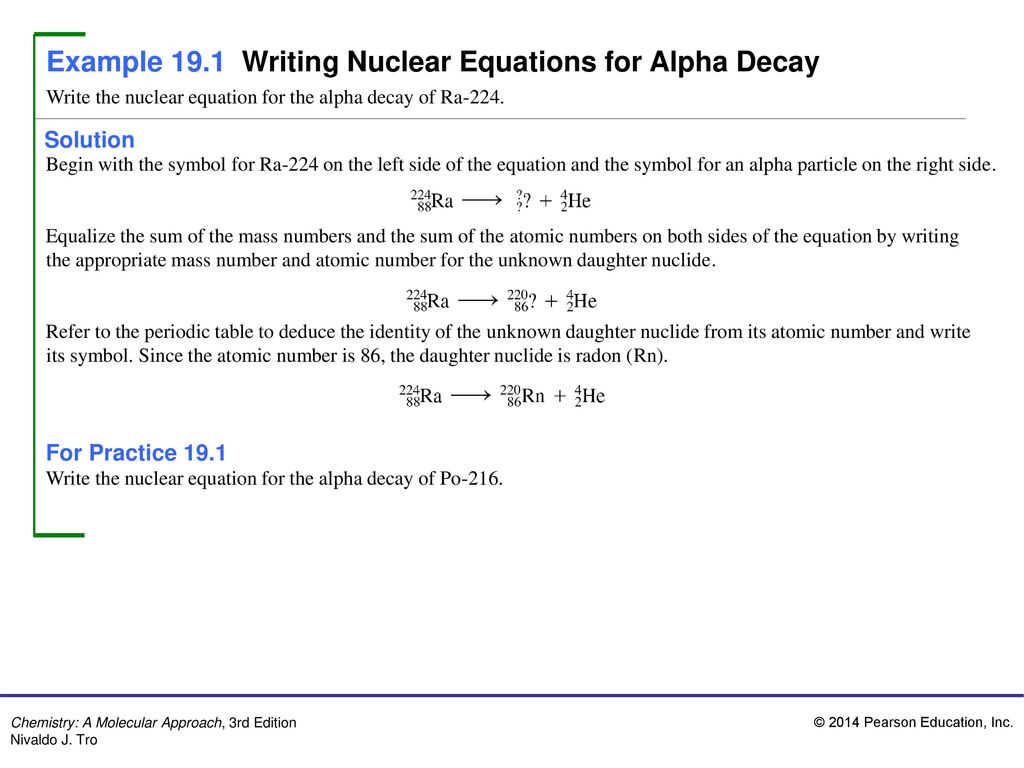
Alpha Decay Equation Example. Write the complete nuclear equation. Nucleon number decreases by 4. Look at the picture for the symbols or molecular representation you will see in the following example decay reactions. This means the number of protons in the nucleus is reduced by 2 and the total number of nucleons is reduced by 4.

24 shows alpha decay of uranium which has six groups of alpha particles in its decay spectrum. Then write down the most basic decay reaction. In alpha decay the unstable isotope will emit an alpha particle along with a more stable isotope or isotopes. The iridium-168 isotope is known to go through alpha decays. The nucleus loses 4 nucleons. 1 and can be found eg.
The energy Q derived from this decay is divided equally into the transformed nucleus and the Helium nucleus.
In alpha decay the unstable isotope will emit an alpha particle along with a more stable isotope or isotopes. Alpha decay helium nucleus 4He 2 or beta decay electron e-or positron e or gamma decay high energy photon. Example 1A typical alpha decay equation. Write out a decay equation that shows this process. Also in nature radioactive nuclides exist that show two different modes of decay. The masses of the elements are conserved during alpha decay.
 Source: wou.edu
Source: wou.edu
So the equation is _84208Po _24He _82204Pb Here is a video that describes how to write equations. This means the number of protons in the nucleus is reduced by 2 and the total number of nucleons is reduced by 4. Proton number decreases by 2. For the following alpha decay equation select the correct isotope product. These correspond to different energy levels of the alpha particles in the U-nucleus see Fig.
 Source: stickmanphysics.com
Source: stickmanphysics.com
1 and can be found eg. EXAMPLE Write a balanced nuclear equation for the α decay of polonium-208. Also in nature radioactive nuclides exist that show two different modes of decay. The nucleus loses 2 protons. It was derived by.
 Source: studylib.net
Source: studylib.net
Also in nature radioactive nuclides exist that show two different modes of decay. The iridium-168 isotope is known to go through alpha decays. So the equation is _84208Po _24He _82204Pb Here is a video that describes how to write equations. In alpha decay an alpha particle is ejected from an unstable nucleus so heres our unstable nucleus uranium-238. ALPHA DECAY A radioactive substance becomes more stable by.
 Source: slidetodoc.com
Source: slidetodoc.com
3 The nucleus left behind has its atomic number reduced by 2 and its mass number reduced by 4 that is by 2 protons and 2 neutrons. β decay is when a neutron turns into a proton. ALPHA DECAY A radioactive substance becomes more stable by. The subscript of X must be 84 2 82. 2 One of these parts the alpha particle goes zooming off into space.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
If a radioactive substance at time t 0 contains N 0 radioactive nuclei then the number of nuclei N remaining after a time interval t is 1 0 N N e Jt where J. The alpha decay of platinum-175 In this reaction platinum-175 undergoes α-decay to produce osmium-171. Alpha decay two protons and two neutrons changes the mass number of. Element 82 is Pb. 3 The nucleus left behind has its atomic number reduced by 2 and its mass number reduced by 4 that is by 2 protons and 2 neutrons.

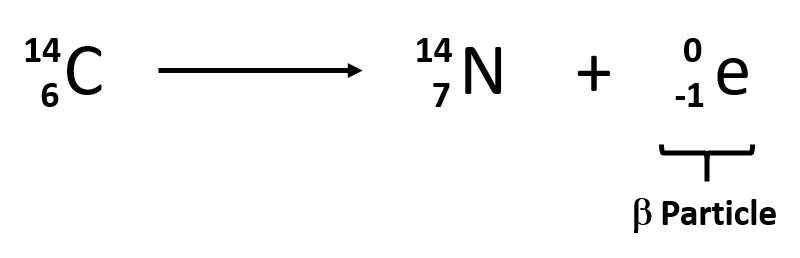
Element 82 is Pb. A beta-minus β particle is a high energy electron emitted from the nucleus. The equation of alpha decay is 217 X N Z A Y N 2 Z 2 A 4 α 2 2 4 Fig. These correspond to different energy levels of the alpha particles in the U-nucleus see Fig. In the equation 14 6 c 14 7 n 0 1 b the decay of radioactive carbon 14 results in the creation of a new nitrogen 14 atom.
 Source: nagwa.com
Source: nagwa.com
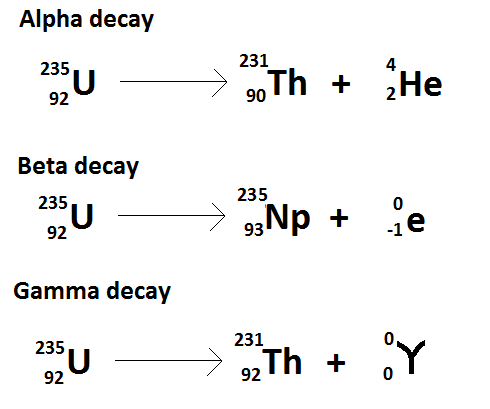
Alpha decay occurs when the nucleus of an atom spontaneously ejects an alpha particle. 1 and can be found eg. So I go ahead and draw in my two neutrons here. Nuclear equations show single alpha and beta decay. 238 92 Ur 234 90 Th4 2He 92 238 Ur 90 234 Th 2 4 He Other examples of alpha decay include.
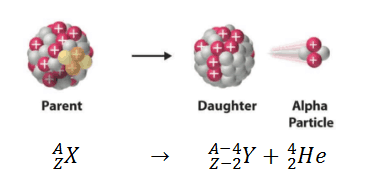 Source: radiation-dosimetry.org
Source: radiation-dosimetry.org
Alpha decay of a nucleus can occur despite the presence of the potential repulsion barrier. Write the complete nuclear equation. Gamows Theory of Geiger-Nutall law defines the relationship between the energy of an alpha particle emitted with the decay constant for a radioactive isotope. A beta-minus β particle is a high energy electron emitted from the nucleus. Proton number decreases by 2.
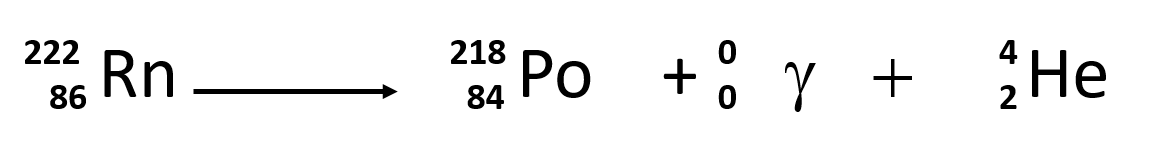 Source: wou.edu
Source: wou.edu
Penetration of Alpha Beta and Gamma Particles. Write the complete nuclear equation. A beta-minus β particle is a high energy electron emitted from the nucleus. Integration of this equation yields N N0eλt where N0 is the size of an initial population of radioactive atoms at time t 0. So I go ahead and draw in my two neutrons here.
 Source: slideshare.net
Source: slideshare.net
The subscript of X must be 84 2 82. Sign in to download full-size image Figure 24. For the following alpha decay equation select the correct isotope product. EXAMPLE Write a balanced nuclear equation for the α decay of polonium-208. The alpha particle is the same as a helium nucleus with 2 protons and 2 neutrons.
 Source: radiation-dosimetry.org
Source: radiation-dosimetry.org
Alpha decay helium nucleus 4He 2 or beta decay electron e-or positron e or gamma decay high energy photon. β decay is when a neutron turns into a proton. Decay constant proportionality between the size of a population of radioactive atoms and the rate at which the population decreases because of radioactive decay. Alpha decay two protons and two neutrons changes the mass number of. The alpha particle is the same as a helium nucleus with 2 protons and 2 neutrons.
 Source: pediaa.com
Source: pediaa.com
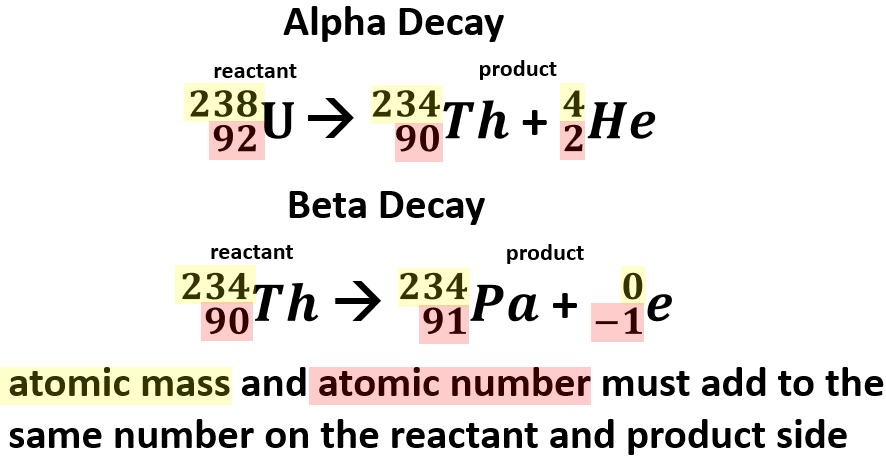
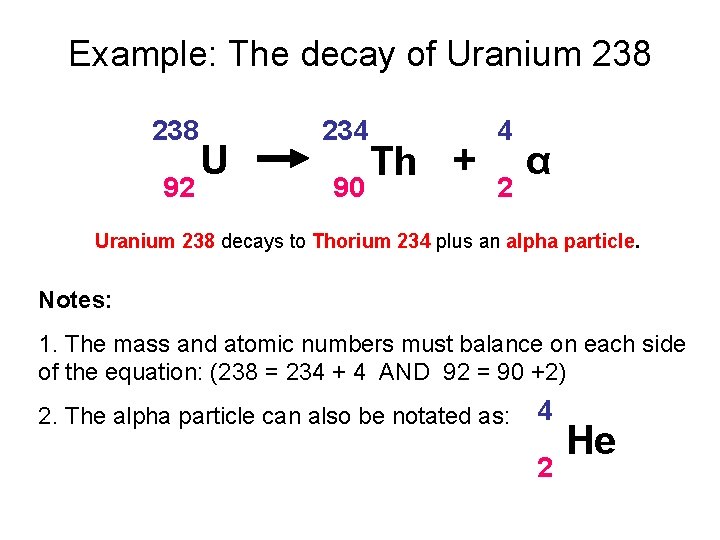
Show what you started with the iridium is your. Nuclear equations show single alpha and beta decay. Alpha Decay Example A well-known example of alpha decay is the decay of uranium 238 92 U 92 238 U to thorium 234 90 Th 90 234 Th with the emission of a helium nucleus 4 2He 2 4 He. Look at the picture for the symbols or molecular representation you will see in the following example decay reactions. Gamows Theory of Geiger-Nutall law defines the relationship between the energy of an alpha particle emitted with the decay constant for a radioactive isotope.

Penetration of Alpha Beta and Gamma Particles. These changes are described using nuclear equations. The alpha decay of uranium-238 In this reaction uranium-238 undergoes α. The nucleus loses 4 nucleons. An example of this decay occurs in the uranium-238 nucleus that decays into thorium-234 nucleus.
 Source: revisionscience.com
Source: revisionscience.com
3 The nucleus left behind has its atomic number reduced by 2 and its mass number reduced by 4 that is by 2 protons and 2 neutrons. 237 93 Np 233 91 Pa4 2He 93 237 Np 91 233 Pa 2 4 He. The subscript of X must be 84 2 82. For example uranium-238 decays to form thorium-234. 2 One of these parts the alpha particle goes zooming off into space.

Write the complete nuclear equation. Write the complete nuclear equation. The equation of alpha decay is 217 X N Z A Y N 2 Z 2 A 4 α 2 2 4 Fig. In alpha decay an alpha particle is ejected from an unstable nucleus so heres our unstable nucleus uranium-238. We saw the helium nucleus in the previous video.
 Source: slideplayer.com
Source: slideplayer.com
One example is presented by 40K that can decay with the emission of a or a particle Fig51. So I go ahead and draw in my two neutrons here. If a radioactive substance at time t 0 contains N 0 radioactive nuclei then the number of nuclei N remaining after a time interval t is 1 0 N N e Jt where J. Start by looking up iridium on your periodic table so that you can find out its atomic number. It was derived by.

One example is presented by 40K that can decay with the emission of a or a particle Fig51. Penetration of Alpha Beta and Gamma Particles. An example of this decay occurs in the uranium-238 nucleus that decays into thorium-234 nucleus. Example 1A typical alpha decay equation. The equation of alpha decay is 217 X N Z A Y N 2 Z 2 A 4 α 2 2 4 Fig.
 Source: youtube.com
Source: youtube.com
Alpha decay of a nucleus can occur despite the presence of the potential repulsion barrier. Challenge nuclear decay worksheet. These correspond to different energy levels of the alpha particles in the U-nucleus see Fig. Nuclear equations show single alpha and beta decay. If a radioactive substance at time t 0 contains N 0 radioactive nuclei then the number of nuclei N remaining after a time interval t is 1 0 N N e Jt where J.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title alpha decay equation example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






