Your Class 1 medical device examples images are ready. Class 1 medical device examples are a topic that is being searched for and liked by netizens today. You can Get the Class 1 medical device examples files here. Download all free photos.
If you’re looking for class 1 medical device examples pictures information linked to the class 1 medical device examples interest, you have visit the ideal site. Our website frequently gives you hints for seeking the maximum quality video and picture content, please kindly search and find more enlightening video content and images that match your interests.
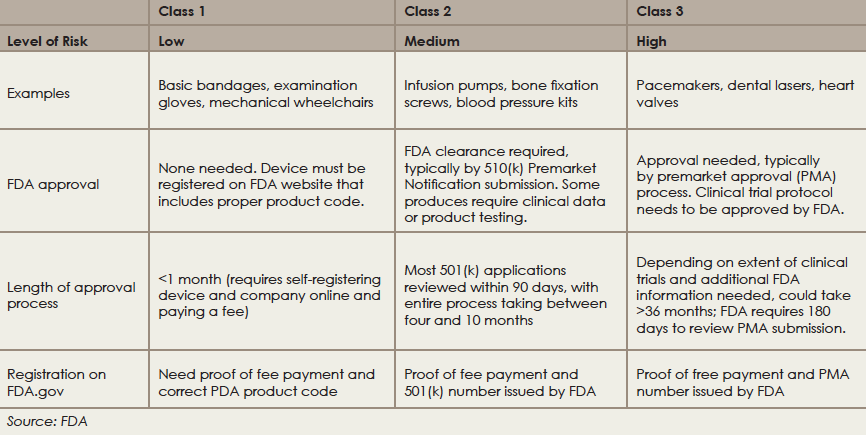
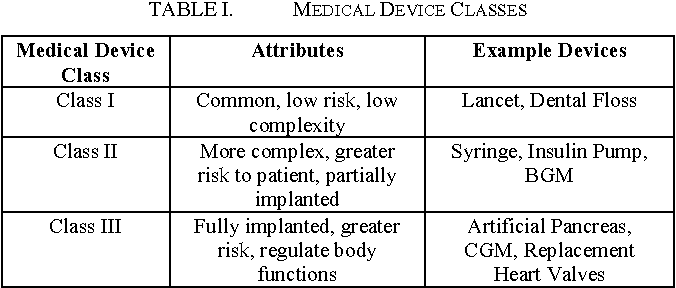
Class 1 Medical Device Examples. These devices help sustain or support life can be implanted andor present potential unreasonable risk of illness or injury. Register with the Competent Authority Vigilance and Post Market Surveillance. This goes from the products with low risk Class I to the products with high risk Class III. Examples include balloon catheters prosthetic heart valves pacemakers etc.
 Iso 10993 1 2018 En Biological Evaluation Of Medical Devices Part 1 Evaluation And Testing Within A Risk Management Process From iso.org
Iso 10993 1 2018 En Biological Evaluation Of Medical Devices Part 1 Evaluation And Testing Within A Risk Management Process From iso.org
Class III medical devices have a high risk to patients or users. Examples of Class 1 medical devices. Elastic bandages dental floss and enemas. They represent about 10 of medical devices and some examples include defibrillators pacemakers breast implants and implanted prosthetics. But if you want to be more specific we can say that there are 3 sub. Choose Conformity Assessment Route.
Authorization of medical devices.
The majority of medical devices are considered to be Class II devices. As we do with drugs medical devices in most countries have to go through a series of review processes before they can enter the marketplace. Compile the Technical File. If you are self-certifying your Class I medical device for the CE mark you will need to meet the requirements of the Medical Devices Regulation 2017745 from 26 May 2021. I share more about types of FDA submissions further on in this guide. The US FDA says that there are three classes of devices in healthcare.

Examples of Class II devices include powered wheelchairs and some pregnancy test kits. Go to article 21 to evaluate if your product is a medical device based on the intended purpose and document the outcome in the technical documentation. Generally speaking class 1 devices have limited contact with patients and their impact on a patients health is slight. Check and confirm that the product is a medical device. In this case of reusable surgical instruments placed on the market the Notified Body involvement is limited to the aspects relating to the re-use of the device in particular cleaning disinfection sterilization maintenance and functional testing and related instructions for use.
![]() Source: akrnconsulting.com
Source: akrnconsulting.com
Identical compliance route to Class IIa devices with an added requirement of a device type examination by a Notified Body. 47 of approved medical devices are Class 1 and 95 of these devices are exempt from the PMA pathway to regulatory approval. Authorization of medical devices. You can find this on the MDR 2017745 to be precise Chapter V Section 1 Article 51. Class 1 2a 2b and 3.
 Source: who.int
Source: who.int
Check and confirm that the product is a medical device. Register with the Competent Authority Vigilance and Post Market Surveillance. Class II medical devices have moderate to higher risks to patients or users. Identical compliance route to Class IIa devices with an added requirement of a device type examination by a Notified Body. Elastic bandages dental floss and enemas.
 Source: enttoday.org
Source: enttoday.org
Refer the flow chart below. As per rule 1 Class 1 is the medical devices either do not touch the body part or just touch the intact skin. Class II medical devices have moderate to higher risks to patients or users. Class 1 device manufacturers are required to register their device with the FDA however. He provides examples of each class and analyses the regulatory and risk implications for each.
 Source: in.pinterest.com
Source: in.pinterest.com
Examples of Class II devices include powered wheelchairs and some pregnancy test kits. Nearly half of all medical devices are class 1 devices covering a wide variety of uses and end users. The MDD 9342EEC defines different rules for the medical devices classification. Medical device licence MDL for Class II III and IV medical devices Class II III or IV medical devices cannot be sold or imported in Canada without a valid medical device licence MDL. Examples of Class 1 medical devices.
The majority of medical devices are considered to be Class II devices. These devices help sustain or support life can be implanted andor present potential unreasonable risk of illness or injury. Class II devices are simple devices though they are more complicated than. Authorization of medical devices. 47 of approved medical devices are Class 1 and 95 of these devices are exempt from the PMA pathway to regulatory approval.
 Source: pinterest.com
Source: pinterest.com
43 of medical devices fall under this category. Authorization of medical devices. In this case of reusable surgical instruments placed on the market the Notified Body involvement is limited to the aspects relating to the re-use of the device in particular cleaning disinfection sterilization maintenance and functional testing and related instructions for use. Nearly half of all medical devices are class 1 devices covering a wide variety of uses and end users. If you are self-certifying your Class I medical device for the CE mark you will need to meet the requirements of the Medical Devices Regulation 2017745 from 26 May 2021.
 Source: twi-global.com
Source: twi-global.com
Class 1 Image created by Market Business News. All Class 1 Medical Devices can affix CE Mark by self-declaration by preparing a Declaration Of Conformity and complying with other requirements. Compile the Technical File. The majority of medical devices are considered to be Class II devices. Class III Medical Devices.
 Source: nursingtimes.net
Source: nursingtimes.net
We refer to these types as low-risk devices. Unlicensed devices that havent been assessed for their safety effectiveness and quality may pose a health risk to Canadians. Go to article 21 to evaluate if your product is a medical device based on the intended purpose and document the outcome in the technical documentation. The EU MDR 2017745 has 4 main categories for Medical Devices classification. According to the EU MDR 2017745 Article 51 medical devices are classified into I IIa IIb and III considering their intended purposes and their inherent risks.
 Source: in.pinterest.com
Source: in.pinterest.com
Class 1 2a 2b and 3. Compile the Technical File. Examples of Class 1 medical devices. Examples of Class II devices include powered wheelchairs and some pregnancy test kits. Class II medical devices have moderate to higher risks to patients or users.
 Source: iso.org
Source: iso.org
If you are self-certifying your Class I medical device for the CE mark you will need to meet the requirements of the Medical Devices Regulation 2017745 from 26 May 2021. Class II devices are simple devices though they are more complicated than. He provides examples of each class and analyses the regulatory and risk implications for each. Examples include ventilators and intensive care monitoring equipment. Examples of Class II devices include powered wheelchairs and some pregnancy test kits.
 Source: pinterest.com
Source: pinterest.com
You can find this on the MDR 2017745 to be precise Chapter V Section 1 Article 51. Class III Devices Class III devices are strictly high risk devices. The US FDA says that there are three classes of devices in healthcare. The risk is incremental from class I to class III. Class 1r medical devices have lowmedium risks perceived.
 Source: in.pinterest.com
Source: in.pinterest.com
These devices help sustain or support life can be implanted andor present potential unreasonable risk of illness or injury. The MDD 9342EEC defines different rules for the medical devices classification. This goes from the products with low risk Class I to the products with high risk Class III. For Class III devices a premarket approval application PMA will be required unless your device is a preamendments device on the market prior to the passage of the medical device amendments in. Or use our MDR Classification Checklist which helps to guide through all the steps.
 Source: pinterest.com
Source: pinterest.com
If you are self-certifying your Class I medical device for the CE mark you will need to meet the requirements of the Medical Devices Regulation 2017745 from 26 May 2021. Class III Medical Devices. Authorization of medical devices. Choose Conformity Assessment Route. Most medical devices are considered Class II devices.
 Source: pinterest.com
Source: pinterest.com
The US FDA says that there are three classes of devices in healthcare. Examples of Class 1 medical devices. The US FDA says that there are three classes of devices in healthcare. Class 1 2a 2b and 3. Class II medical devices have moderate to higher risks to patients or users.
 Source: consteril.com
Source: consteril.com
Examples include ventilators and intensive care monitoring equipment. Class III Devices Class III devices are strictly high risk devices. Steps for Class I medical devices compliance. If you are self-certifying your Class I medical device for the CE mark you will need to meet the requirements of the Medical Devices Regulation 2017745 from 26 May 2021. In this example I learn that my product is a Class II medical device performance standards which means I will need to submit a 510k to FDA prior to getting market clearance.
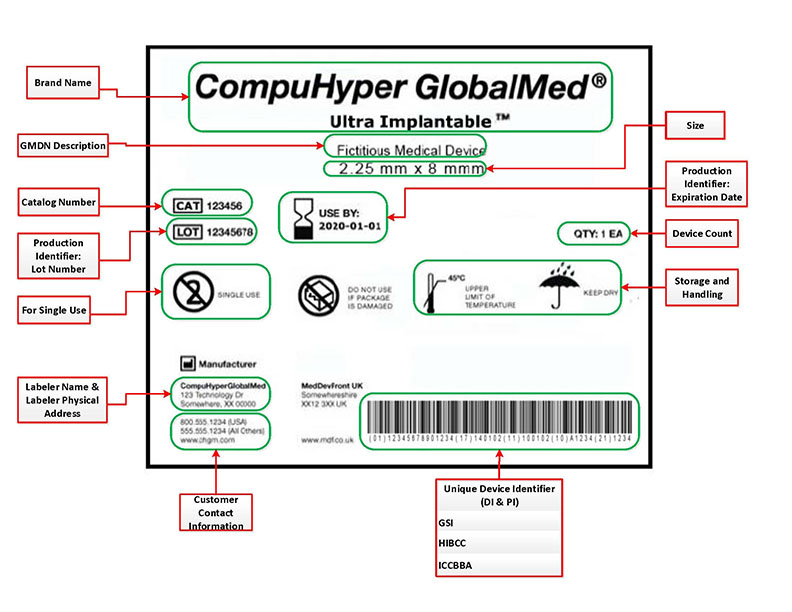
 Source: semanticscholar.org
Source: semanticscholar.org
Or use our MDR Classification Checklist which helps to guide through all the steps. You can find this on the MDR 2017745 to be precise Chapter V Section 1 Article 51. Elastic bandages dental floss and enemas. We refer to these types as low-risk devices. Choose Conformity Assessment Route.
 Source: fda.gov
Source: fda.gov
Examples of Class II devices include powered wheelchairs and some pregnancy test kits. They represent about 10 of medical devices and some examples include defibrillators pacemakers breast implants and implanted prosthetics. The EU MDR 2017745 has 4 main categories for Medical Devices classification. Elastic bandages dental floss and enemas. Based on the class and the rule of the device the technical file and the Notified Body application can be filed.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title class 1 medical device examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






