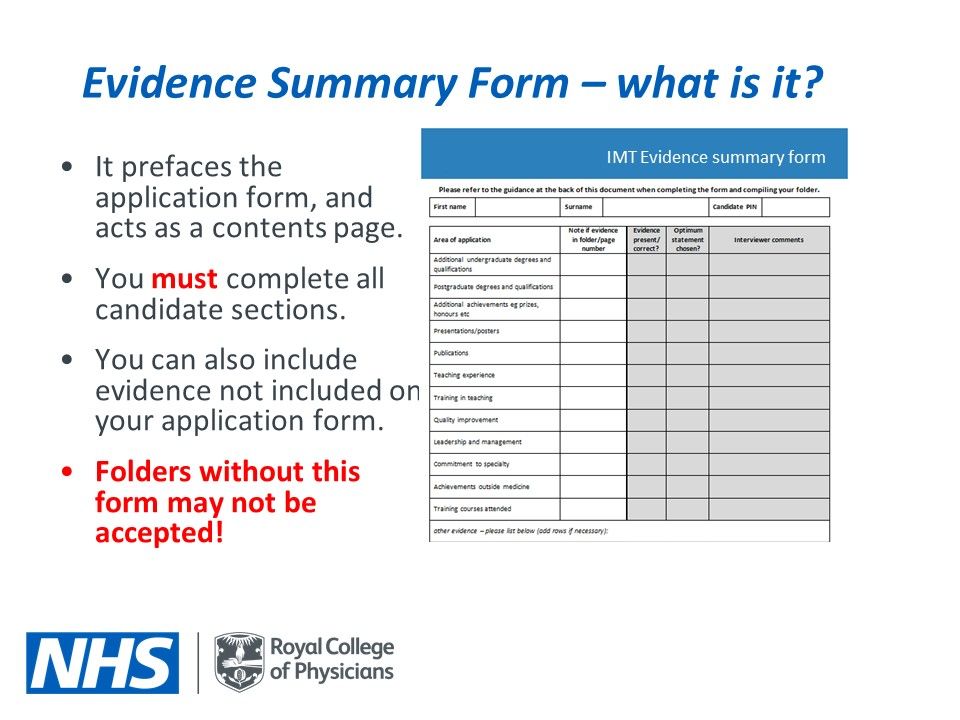
Your Common ion effect example images are ready in this website. Common ion effect example are a topic that is being searched for and liked by netizens now. You can Download the Common ion effect example files here. Get all free photos.
If you’re looking for common ion effect example pictures information connected with to the common ion effect example keyword, you have visit the right blog. Our site frequently provides you with hints for seeking the highest quality video and image content, please kindly surf and locate more informative video content and images that match your interests.
Common Ion Effect Example. Explore New Heights. If you just shook up some solid leadII chloride with water then the solution would. Common ion effect Common ion effect Let us understand the Common ion effect by taking an example. K sp Ag 2 CrO 4 2 90 x 10-12 s 2 0010 x 30 x 10-5 M.
 Pin By Brianna Rico On Crystal Guide Cats Eye Aquamarine Aquamarine Meaning Cats Eye Stone From pinterest.com
Pin By Brianna Rico On Crystal Guide Cats Eye Aquamarine Aquamarine Meaning Cats Eye Stone From pinterest.com
What is the common ion effect. If to an ionic equilibrium AB A B a salt containing a common ion is added the equilibrium shifts in the backward direction. An example of the common ion effect is when sodium chloride NaCl is added to a solution of HCl and water. In a saturated solution of silver chloride we have the equilibrium. Equilibria Involving Complex Ions Complex Ion. The reduction of the degree of dissociation of a salt by the addition of a common-ion is called the common ion effect.
If you just shook up some solid leadII chloride with water then the solution would.
Acetic acid being a weak acid ionizes to a small extent as. LeadII chloride is sparingly soluble in water and this equilibrium is set up between the solid and its ions in solution. What is the common ion effect. Let us take an example of a Silver chloride solution in which equilibrium is achieved. If to an ionic equilibrium AB A B a salt containing a common ion is added the equilibrium shifts in the backward direction. When sodium chloride is added to the solution the concentration of Cl ions will increases.
 Source: pinterest.com
Source: pinterest.com
An example of the common ion effect is when sodium chloride NaCl is added to a solution of HCl and water. The common ion effect causes the reduction of solubility when adding like ions. Common ion effect explaintion of common ion effect with examples11th class chemistrychapter number 8. What is the common ion effect. For example this would be like trying to dissolve solid table salt NaCl in a solution where the chloride ion Cl is already present.
 Source: pinterest.com
Source: pinterest.com
Sodium chloride along with impurities such as Sodium sulphate and Magnesium sulphatecan be purified by applying this principle. EXAMPLE The common ion effect of H 3 O on the ionization of acetic acid. AgCl will be our example. AgC l a q A g C l If we add sodium chloride to this naturally we will get more concentration of Chlorine. Equilibria Involving Complex Ions Complex Ion.
 Source: pinterest.com
Source: pinterest.com
AgCl will be our example. Common ion effect explaintion of common ion effect with example SQ. The dissociation of a weak electrolyte is decreased by adding to the solution a strong electrolyte ie. This effect is called the common ion effect. A salt that has an ion in common with the weak electrolyte Example Acetic acid CH 3 COOH is a weak acid with the following ionization reaction.
 Source: pinterest.com
Source: pinterest.com
CH 3 COOH H 2 O H 3 O CH 3 COO-Ka 18 x 10-5. The reduction of the degree of dissociation of a salt by the addition of a common-ion is called the common ion effect. CH 3 COOH H 2 O H 3 O CH 3 COO-Ka 18 x 10-5. Common ion effect explaintion of common ion effect with examples11th class chemistrychapter number 8. Equilibria Involving Complex Ions Complex Ion.
 Source: pinterest.com
Source: pinterest.com
The reduction of the degree of dissociation of a salt by the addition of a common-ion is called the common ion effect. 1 Concentration of dichromate ion from potassium chromate. EXAMPLE The common ion effect of H 3 O on the ionization of acetic acid. LeadII chloride is slightly soluble in water resulting in the following equilibrium. The equilibrium constants characterizing the stepwise addition of ligands to metal ions.
 Source: pinterest.com
Source: pinterest.com
If to an ionic equilibrium AB A B a salt containing a common ion is added the equilibrium shifts in the backward direction. This is called common ion effect. Adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. The common ion effect is not only used in the quantitative analysis of compounds but is also for their purification. Whenever a solution of an ionic substance comes into contact with another ionic compound with a common ion the solubility of the ionic substance decreases significantly.
 Source: in.pinterest.com
Source: in.pinterest.com
An example of the common ion effect is when sodium chloride NaCl is added to a solution of HCl and water. Dissociation of a Weak Acid. A salt that has an ion in common with the weak electrolyte Example Acetic acid CH 3 COOH is a weak acid with the following ionization reaction. Here the common ion is the chloride ion. Common ion effect Common ion effect Let us understand the Common ion effect by taking an example.
 Source: pinterest.com
Source: pinterest.com
The phenomenon in which the degree of dissociation of any weak electrolyte is suppressed by adding a small amount of strong electrolyte containing a common ion is called a common ion effect. Dissociation of a Weak Acid. Explore New Heights. Adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Example 2 The common ion effect of H 3 O on the ionization of acetic acid.
 Source: pinterest.com
Source: pinterest.com
Explore New Heights. The common ion effect causes the reduction of solubility when adding like ions. Explore New Heights. Gaseous hydrogen chloride is passed through it. The phenomenon in which the degree of dissociation of any weak electrolyte is suppressed by adding a small amount of strong electrolyte containing a common ion is called a common ion effect.
 Source: pinterest.com
Source: pinterest.com
In a saturated solution of silver chloride we have the equilibrium. CH 3 COOH H 2 O H 3 O CH 3 COO-Ka 18 x 10-5. LeadII chloride is sparingly soluble in water and this equilibrium is set up between the solid and its ions in solution. When sodium chloride is added to the solution the concentration of Cl ions will increases. AgCl is an ionic substance and when a tiny bit of it dissolves in solution it dissociates 100 into silver ions Ag and chloride ions Cl.
 Source: pinterest.com
Source: pinterest.com
For example this would be like trying to dissolve solid table salt NaCl in a solution where the chloride ion Cl is already present. This is called common ion effect. The dissociation of a weak electrolyte is decreased by adding to the solution a strong electrolyte ie. AgCl is an ionic substance and when a tiny bit of it dissolves in solution it dissociates 100 into silver ions Ag and chloride ions Cl. Example 2 The common ion effect of H 3 O on the ionization of acetic acid.
 Source: pinterest.com
Source: pinterest.com
Whenever a solution of an ionic substance comes into contact with another ionic compound with a common ion the solubility of the ionic substance decreases significantly. Acetic acid being a weak acid ionizes to a small extent as. An example of the common ion effect is when sodium chloride NaCl is added to a solution of HCl and water. Common Ion Effect Applications problems and examples. If to an ionic equilibrium AB A B a salt containing a common ion is added the equilibrium shifts in the backward direction.
 Source: pinterest.com
Source: pinterest.com
Gaseous hydrogen chloride is passed through it. Dissociation of a Weak Acid. Common Ion Effect on Weak Acids and Bases The common ion effect suppresses the ionization of a weak acid by adding more of an ion that is a product of this equilibrium. The Common Ion Effect Go to Problems 1 - 10 Return to Equilibrium Menu The solubility of insoluble substances can be decreased by the presence of a common ion. What is the common ion effect.
 Source: pinterest.com
Source: pinterest.com
The common ion effect suppresses the ionization of a weak acid by adding more of an ion that is a product of this equilibrium. This effect is called the common ion effect. The common ion effect is not only used in the quantitative analysis of compounds but is also for their purification. 2 Calculate solubility of Ag. Gaseous hydrogen chloride is passed through it.
 Source: pinterest.com
Source: pinterest.com
For example this would be like trying to dissolve solid table salt NaCl in a solution where the chloride ion Cl is already present. LeadII chloride is sparingly soluble in water and this equilibrium is set up between the solid and its ions in solution. If to an ionic equilibrium AB A B a salt containing a common ion is added the equilibrium shifts in the backward direction. Consider a sodium chloride solution. CH3COOH CH3COO H To this solution suppose the salt of this weak acid with a strong base is added.
 Source: pinterest.com
Source: pinterest.com
This is called common Ion effect. The solubility of leadII chloride in water. This excess in the chloride ion leads to the precipitation of NaCl contributed by the dissociation of ions in HCl. 2 Calculate solubility of Ag. The common ion effect is not only used in the quantitative analysis of compounds but is also for their purification.
 Source: pinterest.com
Source: pinterest.com
Common Ion Effect Applications problems and examples. If to an ionic equilibrium AB A B a salt containing a common ion is added the equilibrium shifts in the backward direction. 2 Calculate solubility of Ag. The Common Ion Effect Go to Problems 1 - 10 Return to Equilibrium Menu The solubility of insoluble substances can be decreased by the presence of a common ion. LeadII chloride is slightly soluble in water resulting in the following equilibrium.
 Source: pinterest.com
Source: pinterest.com
Adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Common Ion Effect. EXAMPLE The common ion effect of H 3 O on the ionization of acetic acid. The Common Ion Effect Go to Problems 1 - 10 Return to Equilibrium Menu The solubility of insoluble substances can be decreased by the presence of a common ion. Most common are 6 and 4 Formation Stability Constants.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title common ion effect example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.