Your Conjugate acid and base example images are available in this site. Conjugate acid and base example are a topic that is being searched for and liked by netizens today. You can Get the Conjugate acid and base example files here. Download all free images.
If you’re looking for conjugate acid and base example images information related to the conjugate acid and base example topic, you have come to the right site. Our website frequently gives you suggestions for downloading the highest quality video and image content, please kindly surf and locate more enlightening video content and images that fit your interests.
Conjugate Acid And Base Example. A conjugate acid of a base results when the base accepts a proton. There are 6 that most consider to be the STRONG acids. A conjugate acid is the product that is different from a base by one proton. An example of an acid-base reaction is.
 Conjugate Acids And Bases Acids Are Pretty Basic From acids-are-pretty-basic.weebly.com
Conjugate Acids And Bases Acids Are Pretty Basic From acids-are-pretty-basic.weebly.com
A conjugate acid of a base results when the base accepts a proton. Consider ammonia reacting with water to form an equilibrium with ammonium ions and hydroxide ions. The pH of NaOH lies above 7 and near to 14. A conjugate acid is the product that is different from a base by one proton. The total absorbance A is the sum of the two absorbances for the weak acid and its conjugate base and all of the terms are greater than zero at all pHs. The conjugates will always be listed on the product side of the reaction.
A conjugate acid is the product that is different from a base by one proton.
When this acid donates an. 3 Proton transfer reactions proceed from the stronger acid-base pair to the weaker acid-base pair. NH HCI c. O is dissolved in water the solution turns basic from the reaction of the oxide ion O. NaOH is a strong base in water as it completely dissociates to form hydroxide ions which can accept protons to form water. What is a conjugate acid Example.
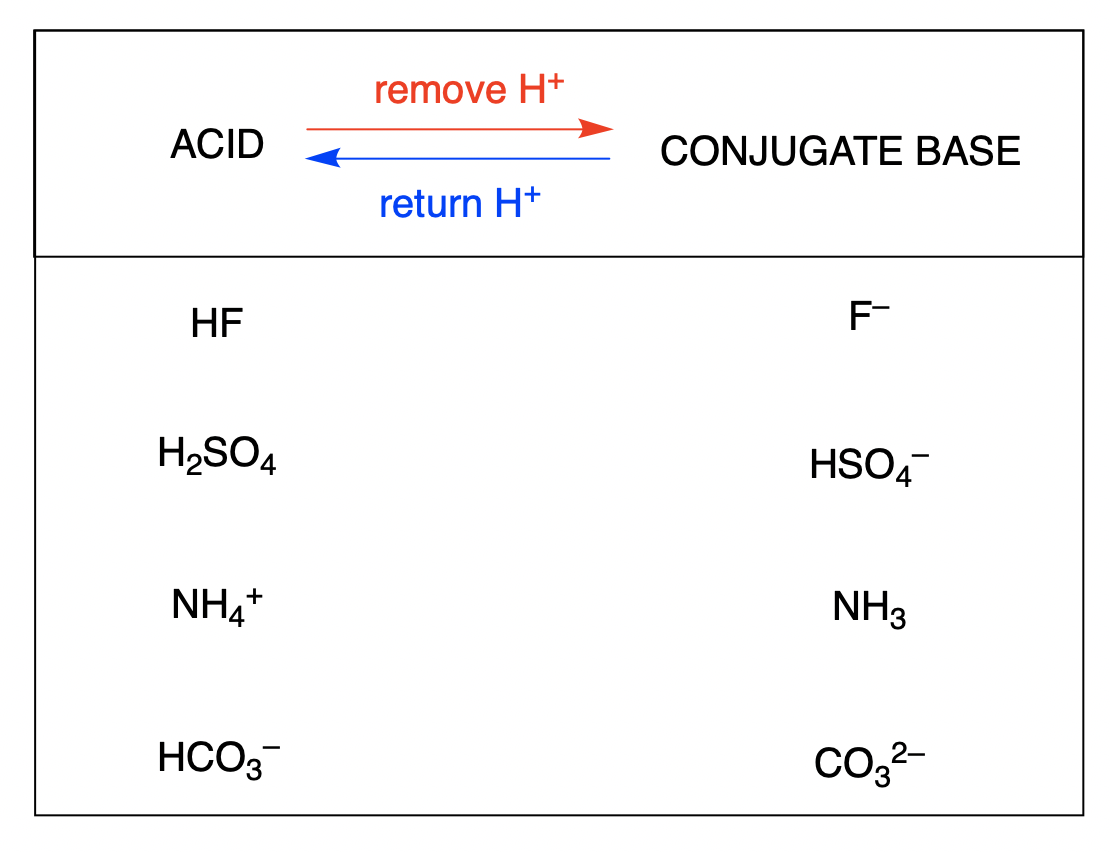 Source: ecampusontario.pressbooks.pub
Source: ecampusontario.pressbooks.pub
A conjugate base is formed when a proton is removed from an acid in a chemical reaction. Acid strength is determined by the amount of that acid that actually ionizes. We explain this with the real world example of vinegarAt Fuse School teachers and animators come together. An example is the base ammonia NH 3 and its conjugate acid the ammonium ion NH 4. A weak base does not dissociate entirely and the pH lies in the range of 7 to 14.
 Source: expii.com
Source: expii.com
HCl HI HBr HNO_3 H_2SO_4 and HClO_4. Thus the equilibria lies on the side of the weaker acid-base pair. Strong acid forms a weak conjugate base and vice versa. H 2 SO 4 H HSO 4. An example is the base ammonia NH 3 and its conjugate acid the ammonium ion NH 4.
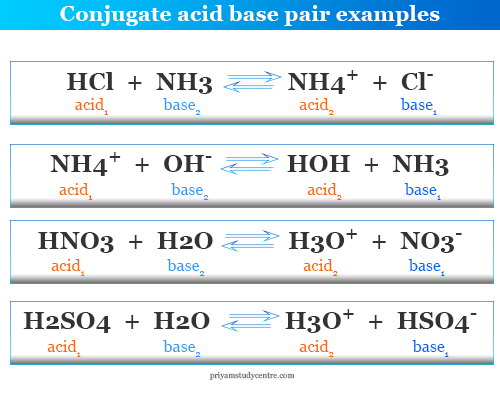 Source: priyamstudycentre.com
Source: priyamstudycentre.com
When a base dissolves in water the species that gains a hydrogen proton is the bases conjugate acid. When a base dissolves in water the species that gains a hydrogen proton is the bases conjugate acid. Identify the acid the base the conjugate acid and the conjugate base in each. NH HCI c. A conjugate acid is the product that is different from a base by one proton.
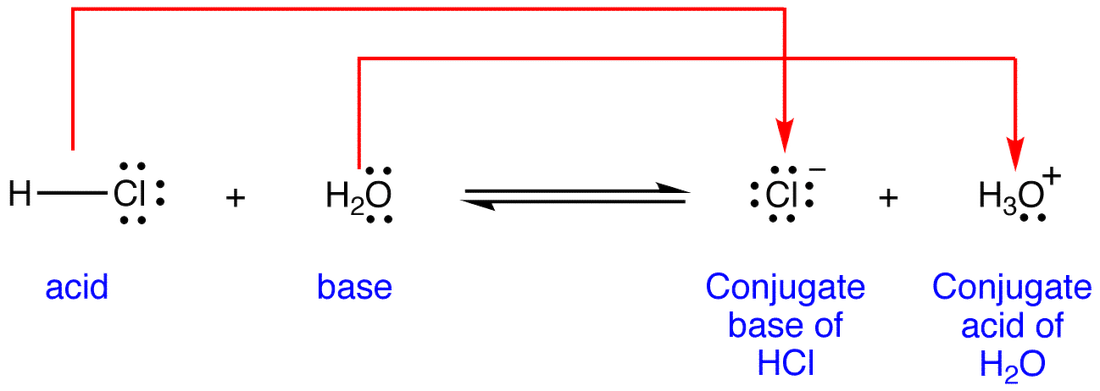 Source: iloveacid–basechemistry.weebly.com
Source: iloveacid–basechemistry.weebly.com
Compare the strength of the conjugate bases for HCl and HC 2H 3O 2. Acid Rewrite each equation. A conjugate acid is the product that is different from a base by one proton. The stronger an acid is the weaker its. There are 6 that most consider to be the STRONG acids.
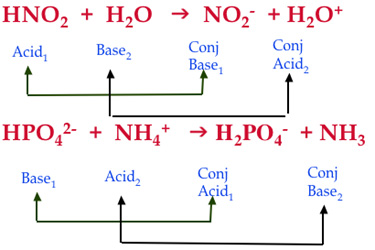 Source: chem2014.weebly.com
Source: chem2014.weebly.com
When does a conjugate acid form in an acid base reaction. Consider ammonia reacting with water to form an equilibrium with ammonium ions and hydroxide ions. All acids have a conjugate base and all bases have a conjugate acid. Acids are molecular covalent compounds which you dont expect to ionize release an H and leave behind the conjugate base or Cl- for example. Compare the strength of the conjugate bases for HCl and HC 2H 3O 2.
 Source: intl.siyavula.com
Source: intl.siyavula.com
An example of an acid-base reaction is. For example aspirin is an acid acetylsalicylic acid and antacids are bases. So the ratio of concentrations must be greater than zero but the right hand side of your last equation has a negative denominator so the quotient is negative. Conjugate acids are a type of acid that is formed when a base accepts a proton in solution. A weak base does not dissociate entirely and the pH lies in the range of 7 to 14.
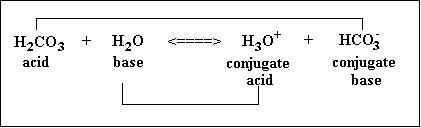 Source: youarebasic.weebly.com
Source: youarebasic.weebly.com
The conjugates will always be listed on the product side of the reaction. Conjugate Acid Definition. We explain this with the real world example of vinegarAt Fuse School teachers and animators come together. The total absorbance A is the sum of the two absorbances for the weak acid and its conjugate base and all of the terms are greater than zero at all pHs. Consider ammonia reacting with water to form an equilibrium with ammonium ions and hydroxide ions.
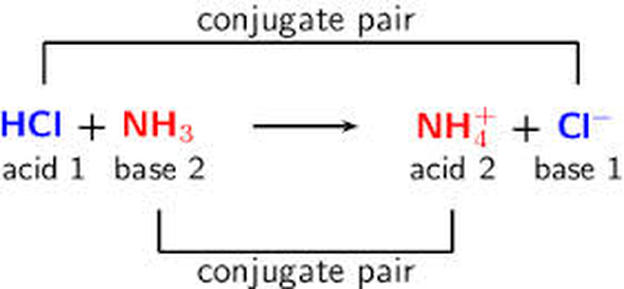 Source: acidsandbases-101.weebly.com
Source: acidsandbases-101.weebly.com
Examples of conjugate acids include water base reacting with an acid to form the hydronium ion conjugate acid and ammonia base reacting with an acid to form the ammonium ion conjugate acid. A conjugate base is formed when a proton is removed from an acid in a chemical reaction. Every time a Brnsted acid acts as an H -ion donor it forms a conjugate baseImagine a generic acid HA. Likewise the stronger the base the weaker its conjugate acid. The conjugates will always be listed on the product side of the reaction.
 Source: study.com
Source: study.com
The stronger a base is the weaker its conjugate acid. 2 aq H. Conjugate acids are a type of acid that is formed when a base accepts a proton in solution. Acid Rewrite each equation. 1417 Identify the relationship between the strength of an acid and its conjugate base and the strength of a base and its conjugate acid The stronger an acid is the weaker its conjugate base.
 Source: youtube.com
Source: youtube.com
Conjugate Acid Definition. A conjugate acid is the product that is different from a base by one proton. 1417 Identify the relationship between the strength of an acid and its conjugate base and the strength of a base and its conjugate acid The stronger an acid is the weaker its conjugate base. The conjugates will always be listed on the product side of the reaction. Acid strength is determined by the amount of that acid that actually ionizes.
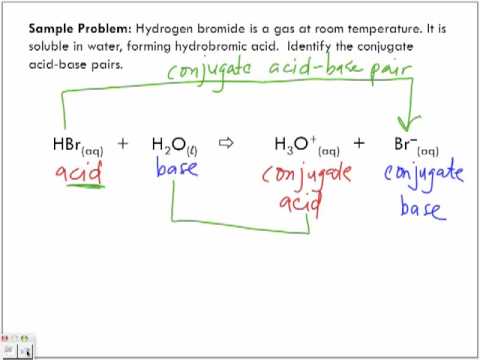 Source: youtube.com
Source: youtube.com
Acidbase reactions are essential in both biochemistry and industrial chemistry. Conjugate bases and conjugate acids are formed in acid-base reactions where an actual acid reacts with an actual base. Acidbase reactions are essential in both biochemistry and industrial chemistry. An acid is converted into its conjugate base by releasing a proton. Conjugate acids and bases are Bronsted-Lowry acid and base pairs determined by which species gains or loses a proton.
 Source: researchgate.net
Source: researchgate.net
Some common examples of conjugate acid-base pairs are HClO 4 H ClO 4. What is a conjugate acid Example. A conjugate base comprises one less H atom and one more negative charge than the acid-forming it. Acids are molecular covalent compounds which you dont expect to ionize release an H and leave behind the conjugate base or Cl- for example. There are 6 that most consider to be the STRONG acids.
 Source: jackwestin.com
Source: jackwestin.com
Any Brønsted acid or base can be thought of as part of a conjugate pair. Examples of conjugate acids include water base reacting with an acid to form the hydronium ion conjugate acid and. The total absorbance A is the sum of the two absorbances for the weak acid and its conjugate base and all of the terms are greater than zero at all pHs. HOCN and OCN - are an example of a conjugate acid-base pair. Begingroup This makes no sense to me.
 Source: acids-are-pretty-basic.weebly.com
Source: acids-are-pretty-basic.weebly.com
When lithium oxide Li. H 2 SO 4 H HSO 4. HCl HI HBr HNO_3 H_2SO_4 and HClO_4. Answer 1 of 3. So the ratio of concentrations must be greater than zero but the right hand side of your last equation has a negative denominator so the quotient is negative.
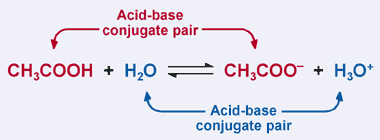 Source: sceweb.sce.uhcl.edu
Source: sceweb.sce.uhcl.edu
Likewise the stronger the base the weaker its conjugate acid. The pH of NaOH lies above 7 and near to 14. NH HCI c. 3 Proton transfer reactions proceed from the stronger acid-base pair to the weaker acid-base pair. The stronger a base is the weaker its conjugate acid.

For example aspirin is an acid acetylsalicylic acid and antacids are bases. HOCN and OCN - are an example of a conjugate acid-base pair. What is a conjugate acid Example. Acid strength is determined by the amount of that acid that actually ionizes. H 2 SO 4 H HSO 4.
 Source: youtube.com
Source: youtube.com
NH HCI c. In other words a conjugate acid is the acid member HX of a pair of compounds that differ. 1417 identify the relationship between the strength. The conjugates will always be listed on the product side of the reaction. Acids and bases exist as conjugate acid-base pairsThe term conjugate comes from the Latin stems meaning joined together and refers to things that are joined particularly in pairs such as Brnsted acids and bases.
 Source: j-tradition.com
Source: j-tradition.com
According to this theory the species that donates a hydrogen cation or proton in a reaction is a conjugate acid while the remaining portion or the one that accepts a proton or hydrogen is the conjugate base. Conjugate bases and conjugate acids are formed in acid-base reactions where an actual acid reacts with an actual base. NH HCI c. All acids have a conjugate base and all bases have a conjugate acid. The conjugate base without the extra proton as the base is in a state to accept a proton.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title conjugate acid and base example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






