Your Conjugate acid base pair examples images are ready. Conjugate acid base pair examples are a topic that is being searched for and liked by netizens now. You can Get the Conjugate acid base pair examples files here. Get all royalty-free photos.
If you’re looking for conjugate acid base pair examples pictures information connected with to the conjugate acid base pair examples topic, you have pay a visit to the ideal blog. Our site frequently gives you suggestions for seeing the highest quality video and picture content, please kindly surf and find more informative video articles and graphics that match your interests.
Conjugate Acid Base Pair Examples. - Voiceover In this video were going to be talking about conjugate acid-base pairs. H 2 O H 2 O H 3 O OH 2 O is OH. While the is the conjugate base. Acids and bases exist as conjugate acid-base pairsThe term conjugate comes from the Latin stems meaning joined together and refers to things that are joined particularly in pairs such as Brnsted acids and bases.
 Chemical Equation Equations Chemical From pinterest.com
Chemical Equation Equations Chemical From pinterest.com
The opposite is true when forming a conjugate base an. The stronger an acid the weaker its conjugate base and conversely the stronger a base the weaker its conjugate acid. Lets focus on the first example CH_3COOH. There are 6 that most consider to be the STRONG acids. NH 3 H 2 O NH 4 OH. In the below picture we provide some examples of conjugated acid-base pairs with their name and formula.
The only difference between the two is a proton H.
Conjugate Acid Definition. Conjugate acid-base pair are compounds which differ by H Heres are two examples of conjugate acid-base pair. 2 with water. H 2 O H 2 O H 3 O OH. In other words a conjugate acid is the acid member HX of a pair of compounds that differ. Conjugate Acid Definition.
 Source: pinterest.com
Source: pinterest.com
Acid strength is determined by the amount of that acid that actually ionizes. Acid base pairs which differed by one proton is called conjugate acid base pair. For example nitric and sulfuric acid easily donates a proton to water to show acid character. Acid Rewrite each equation. A conjugate acid-base pair contains two compounds that differ only by a hydrogen ion H and a charge of 1.
 Source: pinterest.com
Source: pinterest.com
If you are thinking about HA as the acid then A-is its conjugate base. 2 with water. So hydrogen fluoride is a weak acid and when you put it in water it will dissociate partially. The example reaction is between hydrogen fluoride or HF and water. But water easily accepts a proton from sulfuric or nitric acid to shows acid base properties.
 Source: pinterest.com
Source: pinterest.com
All acids have a conjugate base and all bases have a conjugate acid. NH HCI c. Acid strength is determined by the amount of that acid that actually ionizes. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H2 O HSO. Conjugate acid-base pair are compounds which differ by H Heres are two examples of conjugate acid-base pair.
 Source: in.pinterest.com
Source: in.pinterest.com
H 2 O H 2 O H 3 O OH 2 O is OH. A conjugate acid is the product that is different from a base by one proton. Were going to introduce the idea of a conjugate acid-base pair using an example reaction. - Voiceover In this video were going to be talking about conjugate acid-base pairs. The only difference between the two is a proton H.
 Source: pinterest.com
Source: pinterest.com
According to this reaction there is a reaction between water and ammonia which in this case are the acid and base respectively because the water donates the protons o. HCl and Cl Usually HCl is called an acid and Cl is called its conjugate base but that can be reversed if the context calls for it. Conjugate acid-base pair are compounds which differ by H Heres are two examples of conjugate acid-base pair. From the list of moleculeion pairs below click on those that are conjugate acid-base pairs. Important Conjugate Acid-Base Pairs.
 Source: pinterest.com
Source: pinterest.com
So hydrogen fluoride is a weak acid and when you put it in water it will dissociate partially. The conjugates will always be listed on the product side of the reaction. In other words a conjugate acid is the acid member HX of a pair of compounds that differ. HOCN and OCN-are an example of a conjugate acid-base pair. The concept of conjugate acid-base pair is related to Bronsted-Lowry acid-base theory and according to this theory acid is a proton H donor while base is a proton acceptor.
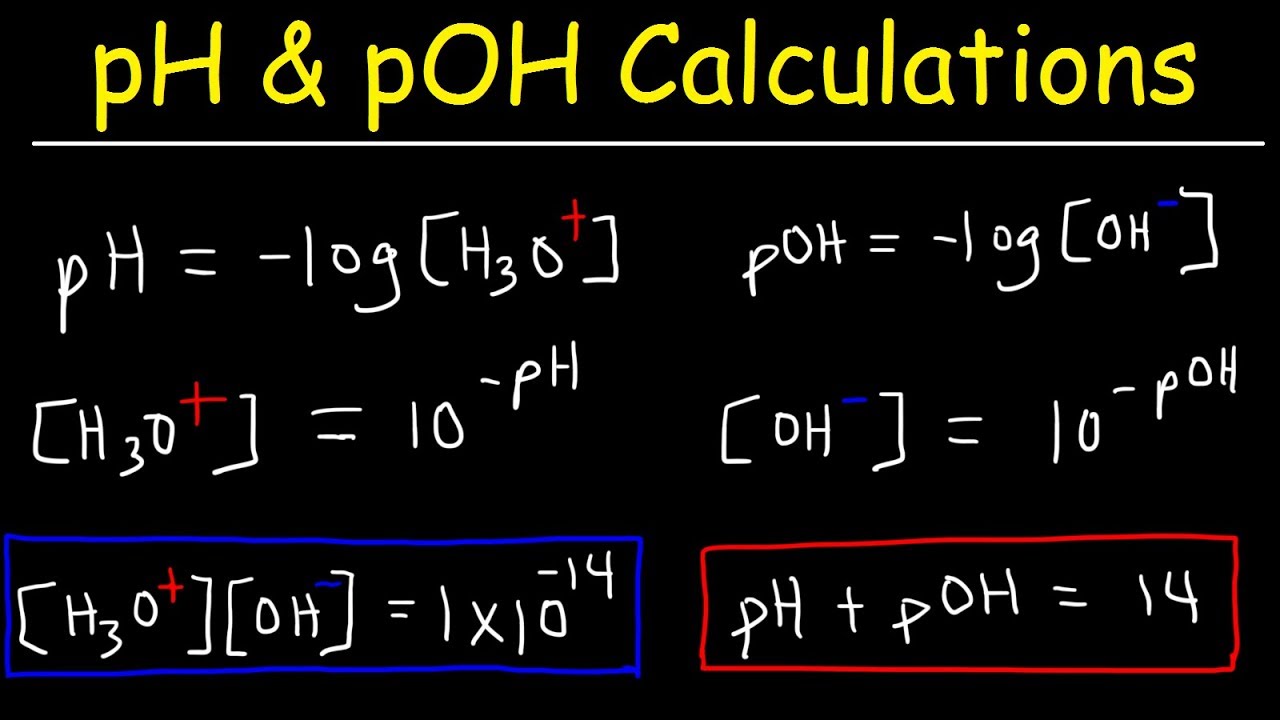 Source: pinterest.com
Source: pinterest.com
Conjugate acid-base pair are compounds which differ by H Heres are two examples of conjugate acid-base pair. All acids have a conjugate base and all bases have a conjugate acid. Conjugate acids and bases are Bronsted-Lowry acid and base pairs determined by which species gains or loses a proton. A conjugate acid is the product that is different from a base by one proton. Members of a conjugate pair differ from each other by the presence or absence of the transferable hydrogen ion.
 Source: pinterest.com
Source: pinterest.com
According to this reaction there is a reaction between water and ammonia which in this case are the acid and base respectively because the water donates the protons o. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H2 O HSO. In both cases identify the conjugate acid base pairs. The concept of conjugate acid-base pair is related to Bronsted-Lowry acid-base theory and according to this theory acid is a proton H donor while base is a proton acceptor. The stronger an acid the weaker its conjugate base and conversely the stronger a base the weaker its conjugate acid.
 Source: in.pinterest.com
Source: in.pinterest.com
The only difference between the two is a proton H. 2 aq H. The opposite is true when forming a conjugate base an. A conjugate acid is formed when a base gains a positive hydrogen Ion H and thus having the ability to lose this ion becomes a weak acid. The conjugates will always be listed on the product side of the reaction.
 Source: in.pinterest.com
Source: in.pinterest.com
OH is. H 2 O H 2 O H 3 O OH. When a base dissolves in water the species that gains a hydrogen proton is the bases conjugate acid. On the other hand a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. The water and the hydroxonium ion are also a conjugate pair.
 Source: in.pinterest.com
Source: in.pinterest.com
O is dissolved in water the solution turns basic from the reaction of the oxide ion O. A conjugate acid is formed when a base gains a positive hydrogen Ion H and thus having the ability to lose this ion becomes a weak acid. Acid strength is determined by the amount of that acid that actually ionizes. Conjugate acid-base pair are compounds which differ by H Heres are two examples of conjugate acid-base pair. Write the reaction that occurs and identify the conjugate acid base pairs.
 Source: pinterest.com
Source: pinterest.com
Conjugate acid base pair examples. Conjugate acid base pair examples. A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion. The concept of conjugate acid-base pair is related to Bronsted-Lowry acid-base theory and according to this theory acid is a proton H donor while base is a proton acceptor. NH 3 H 2 O NH 4 OH.
 Source: pinterest.com
Source: pinterest.com
When lithium oxide Li. NH HCI c. A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion. When lithium oxide Li. In both cases identify the conjugate acid base pairs.
 Source: pinterest.com
Source: pinterest.com
- Voiceover In this video were going to be talking about conjugate acid-base pairs. Important Conjugate Acid-Base Pairs. Example Wiitc an equation that shows NH3 reacting with HCL Label the acid base and conjugate acid and conjugate base Write reactants and transfer a proton from the acid to the base. If you are thinking about A-as the base then HA is its conjugate acid. - Voiceover In this video were going to be talking about conjugate acid-base pairs.
 Source: pinterest.com
Source: pinterest.com
Conjugate base of acid H 2 O is OH. The strongest acids ionize 100. NH HCI c. A conjugate acid is the product that is different from a base by one proton. If you are thinking about HA as the acid then A-is its conjugate base.
 Source: pinterest.com
Source: pinterest.com
The water and the hydroxonium ion are also a conjugate pair. HCl HI HBr HNO_3 H_2SO_4 and HClO_4. A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion. The water and the hydroxonium ion are also a conjugate pair. Conjugate Acid Definition.
 Source: pinterest.com
Source: pinterest.com
When lithium oxide Li. The conjugates will always be listed on the product side of the reaction. Some common examples of conjugate acid-base pairs are HClO 4 H ClO 4. O is dissolved in water the solution turns basic from the reaction of the oxide ion O. From the list of moleculeion pairs below click on those that are conjugate acid-base pairs.
 Source: pinterest.com
Source: pinterest.com
According to this reaction there is a reaction between water and ammonia which in this case are the acid and base respectively because the water donates the protons o. From the list of moleculeion pairs below click on those that are conjugate acid-base pairs. It behaves as an acid. A conjugate acid is the product that is different from a base by one proton. A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title conjugate acid base pair examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






