Your Dipole induced dipole example images are ready. Dipole induced dipole example are a topic that is being searched for and liked by netizens today. You can Find and Download the Dipole induced dipole example files here. Get all free images.
If you’re looking for dipole induced dipole example pictures information linked to the dipole induced dipole example topic, you have come to the ideal blog. Our site always provides you with suggestions for seeking the highest quality video and picture content, please kindly search and find more informative video content and graphics that fit your interests.
Dipole Induced Dipole Example. This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole. Neither dipole-dipole nor dipole-induced forces can explain the fact that helium becomes a liquid at temperatures below 42 K. What is induced dipole. A permanent magnet is magnetic because of the magnetic dipole moment of the electron.
 Induced Dipole Forces From chem.purdue.edu
Induced Dipole Forces From chem.purdue.edu
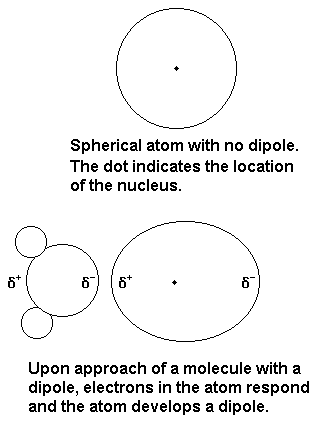
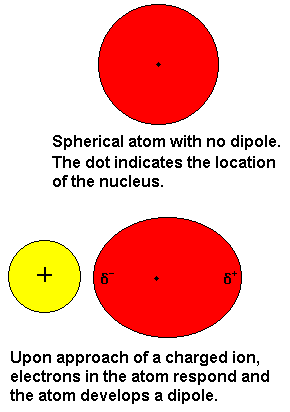
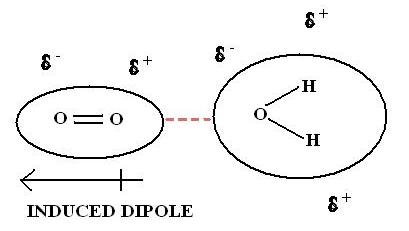
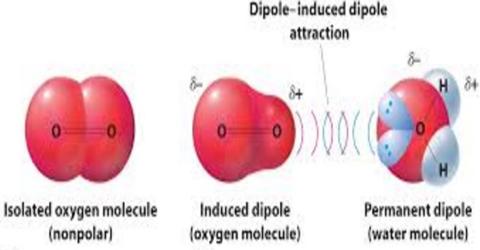
Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules. Declare the difference between linked and non-bonded attractions. An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. For example a water molecule H2O is a dipole. All biopolymers are chiral and directional with distinctive ends. By itself a helium atom is perfectly symmetrical.
Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules.
What is induced dipole. What is dipole-induced dipole forces give an example. Dipole vs Induced Dipole For example H2O is a dipole as the hydrogens have a partial positive charge and the oxygen has a partial negative charge. Polar molecules can also induce dipoles in nonpolar molecules resulting in dipoleinduced dipole forces. All biopolymers are chiral and directional with distinctive ends. For molecules of similar size and mass the strength of these forces increases with increasing polarity.
 Source: chem.purdue.edu
Source: chem.purdue.edu
What is induced dipole. By itself a helium atom is perfectly symmetrical. Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. Polar molecules can also induce dipoles in nonpolar molecules resulting in dipoleinduced dipole forces. These are weak forces.
 Source: chemistrylearner.com
Source: chemistrylearner.com
But if it is polar then this interaction augments the dipoledipole interaction described above. Dipole vs Induced Dipole For example H2O is a dipole as the hydrogens have a partial positive charge and the oxygen has a partial negative charge. While both are used to hold chemical systems together they each introduce. A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. The various sugars are important example of ion induced dipole interaction between water molecule and accepting two nonmetals that forces.
 Source: toppr.com
Source: toppr.com
This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole. Temporary dipole attractions between nonpolar molecules that form due to shifting electrons. Declare the difference between linked and non-bonded attractions. In instantaneous dipole-induced dipole the atoms or molecules electrons are in spontaneous presence around the electron orbital causing fluctuations in their polarity structureOnce a dipole is spontaneously created the incident will induce a polarity in another neighboring molecule. Examples of ion induced dipole forces.
 Source: j-tradition.com
Source: j-tradition.com
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. These forces are of short range and small magnitude. A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. Sometimes called induced dipole forces or just dispersion forces.
 Source: youtube.com
Source: youtube.com
Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. What is dipole-induced dipole forces give an example. Since both molecules are non-polar all the bond dipoles cancel out the only. This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole. One example of a dipole antenna is the old style TV rabbit ears antenna which is a folded dipoleA dipole antenna is any antenna with 2 active elements.
 Source: learnbiochemistry.wordpress.com
Source: learnbiochemistry.wordpress.com
The second participating molecule need not be polar. Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. O London Dispersion Forces LDF. Examples of ion induced dipole forces. But if it is polar then this interaction augments the dipoledipole interaction described above.
 Source: slideplayer.com
Source: slideplayer.com
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. The dipoleinduced-dipole interaction depends on the presence of a. But if it is polar then this interaction augments the dipoledipole interaction described above. This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole.

A permanent magnet is magnetic because of the magnetic dipole moment of the electron. Examples of ion induced dipole forces. An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. What is dipole induced dipole forces give an example Induced-Dipole Forces Induced dipole forces result when an ion or a dipole induces a dipole in an atom or a molecule with no dipole. Dipole vs Induced Dipole For example H2O is a dipole as the hydrogens have a partial positive charge and the oxygen has a partial negative charge.
 Source: researchgate.net
Source: researchgate.net
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. Oxygen doubling the charge of both. Declare the difference between linked and non-bonded attractions. A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. A permanent magnet is magnetic because of the magnetic dipole moment of the electron.
 Source: emedicalprep.com
Source: emedicalprep.com
Polar molecules can also induce dipoles in nonpolar molecules resulting in dipoleinduced dipole forces. For molecules of similar size and mass the strength of these forces increases with increasing polarity. A permanent magnet is magnetic because of the magnetic dipole moment of the electron. If a liquid is cooled tooquickly blue. All molecules have induced dipole-induced dipole moments but LDFs London dispersion forces the same thing as induced dipole-induced dipole moments is most significant for interactions between nonpolar molecules.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Ion induced dipole forces examples One of the biggest sources of difficulty for a chemistry student is the distinction between chemical bonds and intermolecular forces. Neither dipole-dipole nor dipole-induced forces can explain the fact that helium becomes a liquid at temperatures below 42 K. Learning targets The name of five types of molecular units that condensed matter can be composed. The various sugars are important example of ion induced dipole interaction between water molecule and accepting two nonmetals that forces. Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules.
 Source: qsstudy.com
Source: qsstudy.com
Ion induced dipole forces examples One of the biggest sources of difficulty for a chemistry student is the distinction between chemical bonds and intermolecular forces. Dipole induced dipole forces examples One of the biggest sources of difficulty for a chemistry student is the distinction between chemical bonds and intermolecular forces. Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. For molecules of similar size and mass the strength of these forces increases with increasing polarity. A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species.
 Source: toppr.com
Source: toppr.com
Ion-Induced Dipole Forces An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole. A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. The dipoleinduced-dipole interaction depends on the presence of a. Low-dimensional systems of dipoles show many interesting features in terms of both spectra and density distributions.
 Source: toppr.com
Source: toppr.com
If a liquid is cooled tooquickly blue. All molecules have induced dipole-induced dipole moments but LDFs London dispersion forces the same thing as induced dipole-induced dipole moments is most significant for interactions between nonpolar molecules. Examples of ion induced dipole forces. While both are used to hold chemical systems together they each introduce. While both are used to hold chemical systems together they each introduce.
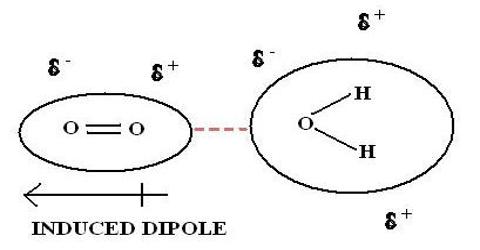 Source: qsstudy.com
Source: qsstudy.com
A permanent magnet is magnetic because of the magnetic dipole moment of the electron. Declare the difference between linked and non-bonded attractions. Low-dimensional systems of dipoles show many interesting features in terms of both spectra and density distributions. Neither dipole-dipole nor dipole-induced forces can explain the fact that helium becomes a liquid at temperatures below 42 K. Learning targets The name of five types of molecular units that condensed matter can be composed.
 Source: sciencedirect.com
Source: sciencedirect.com
But CF2Cl2 has tetrahedral geometry which is not symmetrical so the dipole charges do not cancel out each other and the molecule has a net dipole moment which. HBr and HF The dipole - dipole forces depend on the orientation of the interacting molecules dipole moments The molecule reorient themselves to maximize the attractive forces Coulombs law A positive pole of one dipole is attracted by a negative pole of another dipole. The above ideas of intermolecular force are unable to explain why molecules which do not have. In instantaneous dipole-induced dipole the atoms or molecules electrons are in spontaneous presence around the electron orbital causing fluctuations in their polarity structureOnce a dipole is spontaneously created the incident will induce a polarity in another neighboring molecule. By itself a helium atom is perfectly symmetrical.
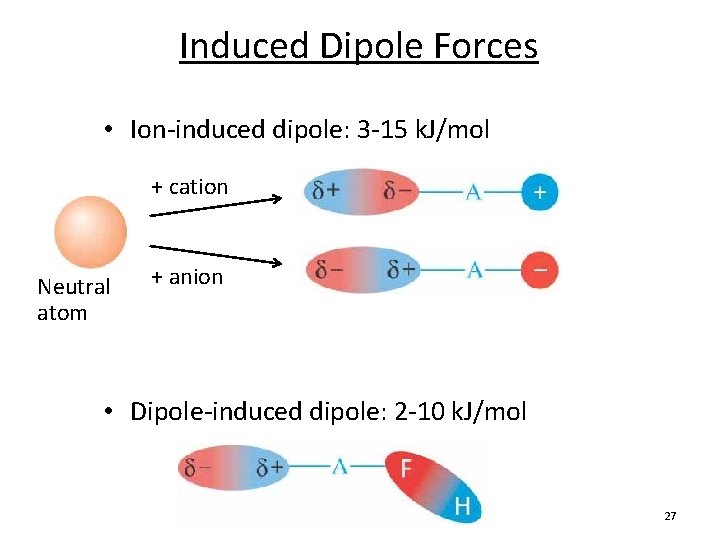 Source: slidetodoc.com
Source: slidetodoc.com
For example CH 4 interacting with another CH 4. Many external parameters of such a system can in fact be controlled in experiments so simulations are invaluable in order to. What is induced dipole. A permanent magnet is magnetic because of the magnetic dipole moment of the electron. Dipoleinduced-dipole interactiontype of attractive interaction the dipoleinduced-dipole interaction also depends on the presence of a polar molecule.
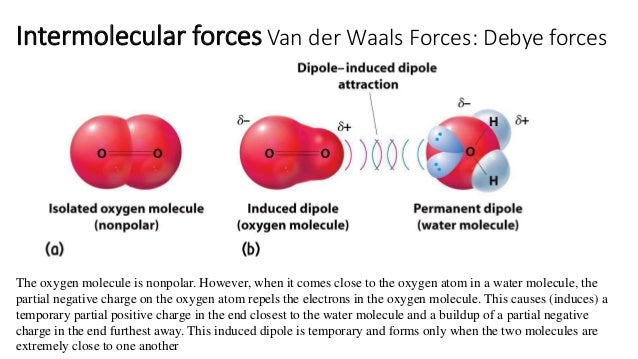 Source: slideshare.net
Source: slideshare.net
Therefore CH2Cl2 interacts with H2O via dipole-dipole forces while CCl4 only interacts with water via dipoleinduced dipole forces or LDFs which would be weaker. For molecules of similar size and mass the strength of these forces increases with increasing polarity. HBr and HF The dipole - dipole forces depend on the orientation of the interacting molecules dipole moments The molecule reorient themselves to maximize the attractive forces Coulombs law A positive pole of one dipole is attracted by a negative pole of another dipole. For example a water molecule H2O is a dipole. This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title dipole induced dipole example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






