Your Heisenberg uncertainty principle example images are ready in this website. Heisenberg uncertainty principle example are a topic that is being searched for and liked by netizens now. You can Get the Heisenberg uncertainty principle example files here. Find and Download all royalty-free images.
If you’re looking for heisenberg uncertainty principle example pictures information linked to the heisenberg uncertainty principle example keyword, you have pay a visit to the right site. Our site frequently provides you with hints for downloading the highest quality video and image content, please kindly surf and find more informative video content and graphics that fit your interests.
Heisenberg Uncertainty Principle Example. The Standard Example. Flexible Online Learning at Your Own Pace. Heisenbergs uncertainty principle states that at any given point in time either position or momentum can only be measured accurately. Heisenberg Uncertainty Principle Problems.
 Heisenberg Uncertainty Principle Defined As Delta X Delta P X Geq H Versus Delta X Delta P X Geq Hbar 2 Physics Stack Exchange From physics.stackexchange.com
Heisenberg Uncertainty Principle Defined As Delta X Delta P X Geq H Versus Delta X Delta P X Geq Hbar 2 Physics Stack Exchange From physics.stackexchange.com
Flexible Online Learning at Your Own Pace. Of a microparticle coordinate and the projection of its impulse along the coordinate axis as well as the uncertainty relation of microparticle time and energy falls into the ranks of the most important quantum relations that express the known uncertainty principle in a mathematical form. Chemistry Quantum Mechanical Model of the Atom Heisenberg Uncertainty Principle. We identified it from obedient source. 21 Heisenberg Uncertainty Principle. The Heisenberg Uncertainty Principle is one example of our inability to have all possible information on a system.
Heisenberg Uncertainty Principle Problems.
It says that some of the properties of atoms and the components of atoms have an inherent fuzziness. Uncertainty in momentum uncertainty in position. The Standard Example. Let us begin it. Very roughly it states that if we know everything about where a particle is located the uncertainty of position is small we know nothing about its momentum the uncertainty of momentum is large and vice versa. Known numerics are v 20 ms m 05 kg h 662607004 10-34 m 2 kg s.
 Source: youtube.com
Source: youtube.com
The more accurately you know the position ie the smaller Δx is the less accurately you know the momentum ie the larger Δp is. Heisenberg Uncertainty Principle Formula. Here Δq is the uncertainty in the position of the particle in metres Δv is the. Very roughly it states that if we know everything about where a particle is located the uncertainty of position is small we know nothing about its momentum the uncertainty of momentum is large and vice versa. Well go through the questions of the Heisenberg Uncertainty principle.
 Source: careerstoday.in
Source: careerstoday.in
The degree of uncertainty can be calculated according to the Heisenberg Uncertainty principle. As we know that P mv 0520 10kg. Active 2 years 2 months ago. Here are a number of highest rated Heisenberg Uncertainty Principle Formula pictures on internet. Electron spins around the orbital near speed of light.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
For the example given earlier Heisenbergs principle can be precisely stated as. Heisenberg s Uncertainty Principle. Heisenberg Uncertainty Principle Problems. Electron spins around the orbital near speed of light. For the example given earlier Heisenbergs principle can be precisely stated as.

Heisenbergs uncertainty principle is a key principle in quantum mechanics. The more accurately the position is known the less precisely its momentum¹ can be determined and vice versa. Known numerics are v 20 ms m 05 kg h 662607004 10-34 m 2 kg s. So for an observer if he calculates the. The Standard Example.
 Source: exactls.com
Source: exactls.com
Heisenbergs uncertainty principle states that at any given point in time either position or momentum can only be measured accurately. Uncertainty in momentum uncertainty in position. We identified it from obedient source. 1 Answer Namikaze R. Of a microparticle coordinate and the projection of its impulse along the coordinate axis as well as the uncertainty relation of microparticle time and energy falls into the ranks of the most important quantum relations that express the known uncertainty principle in a mathematical form.
 Source: youtube.com
Source: youtube.com
As we know that P mv 0520 10kg. In 1927 the German physicist Werner Heisenberg put forth what has become known as the Heisenberg uncertainty principle or just uncertainty principle or sometimes Heisenberg principleWhile attempting to build an intuitive model of quantum physics Heisenberg had uncovered that there were certain fundamental relationships which put. Here are a number of highest rated Heisenberg Uncertainty Principle Formula pictures on internet. The Heisenberg Uncertainty Principle HUP is also called the Indeterminacy Principle or simply the Uncertainty Principle. Viewed 490 times 3 begingroup Beside momentum and position I read that Heisenberg Uncertainty can also apply to energy and time so for instance virtual particle energy could pop into existence but for a very.
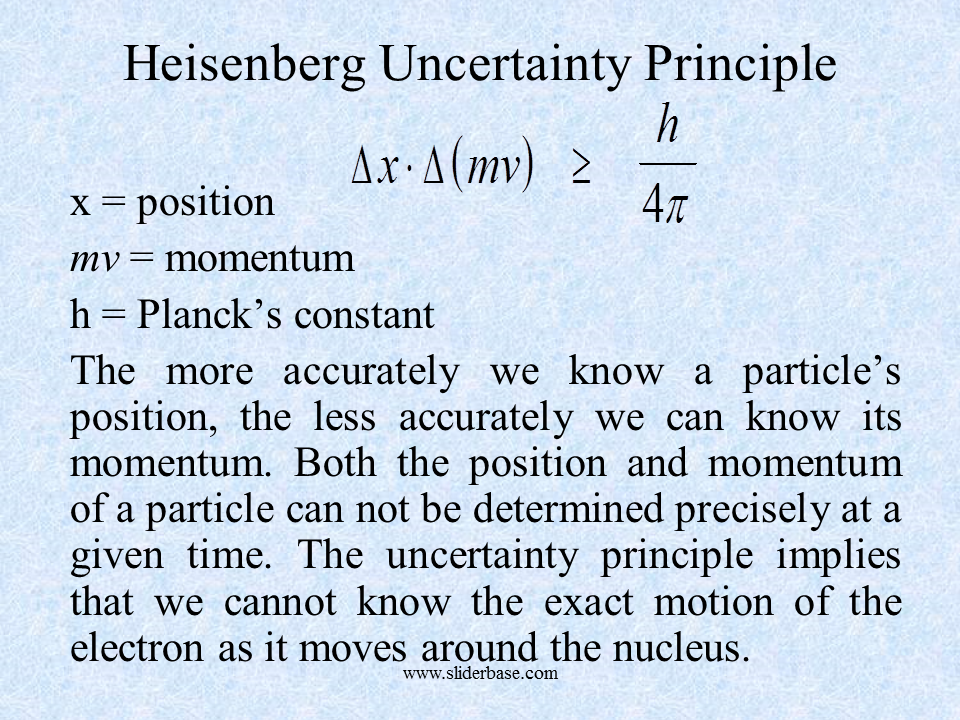
The Heisenberg Uncertainty Principle is one example of our inability to have all possible information on a system. For example it is not possible to discover with precision both the momentum and the position of a quantum. The Standard Example. The degree of uncertainty can be calculated according to the Heisenberg Uncertainty principle. Flexible Online Learning at Your Own Pace.
 Source: youtube.com
Source: youtube.com
Active 2 years 2 months ago. The uncertainty principle played an important role in many discussions on the philosophical implications of quantum mechanics in particular in discussions on the consistency of the so-called Copenhagen interpretation the interpretation endorsed by the founding fathers Heisenberg and Bohr. Mathematically the Heisenberg uncertainty. As mentioned the security of quantum cryptography rests on several principles from quantum physics. Solved Examples for Heisenberg Uncertainty Formula.
 Source: youtube.com
Source: youtube.com
This principle was given in 1927 by the German physicist Werner Heisenberg. The Standard Example. Δp is the uncertainty in momentum. This article will discuss the uncertainty principle as well Uncertainty Principle formula with examples. Ad Build your Career in Data Science Web Development Marketing More.

For the example given earlier Heisenbergs principle can be precisely stated as. The Heisenbergs uncertainty relation. Here are a number of highest rated Heisenberg Uncertainty Principle Formula pictures on internet. It says that some of the properties of atoms and the components of atoms have an inherent fuzziness. Jan 5 2016 like electrons momentum and position.
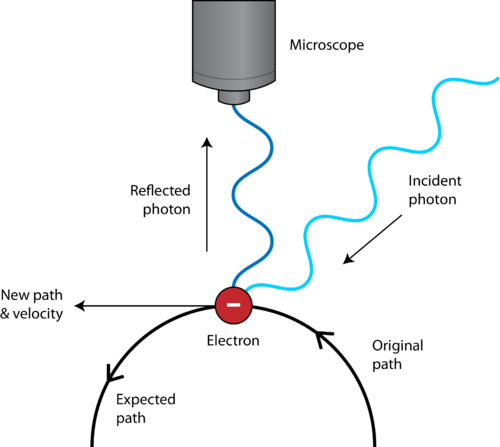 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Δx is the uncertainty in position. The Heisenberg Uncertainty Principle HUP is also called the Indeterminacy Principle or simply the Uncertainty Principle. The more accurately you know the position ie the smaller Δx is the less accurately you know the momentum ie the larger Δp is. Heisenbergs uncertainty principle states that at any given point in time either position or momentum can only be measured accurately. Its submitted by admin in the best field.
 Source: youtube.com
Source: youtube.com
As mentioned the security of quantum cryptography rests on several principles from quantum physics. The most fundamental of these principles is the Heisenberg Uncertainty Principle HUP which states that in a quantum system only one property of a pair of conjugate properties can be known with certainty. Very roughly it states that if we know everything about where a particle is located the uncertainty of position is small we know nothing about its momentum the uncertainty of momentum is large and vice versa. Chemistry Quantum Mechanical Model of the Atom Heisenberg Uncertainty Principle. In 1927 the German physicist Werner Heisenberg put forth what has become known as the Heisenberg uncertainty principle or just uncertainty principle or sometimes Heisenberg principleWhile attempting to build an intuitive model of quantum physics Heisenberg had uncovered that there were certain fundamental relationships which put.
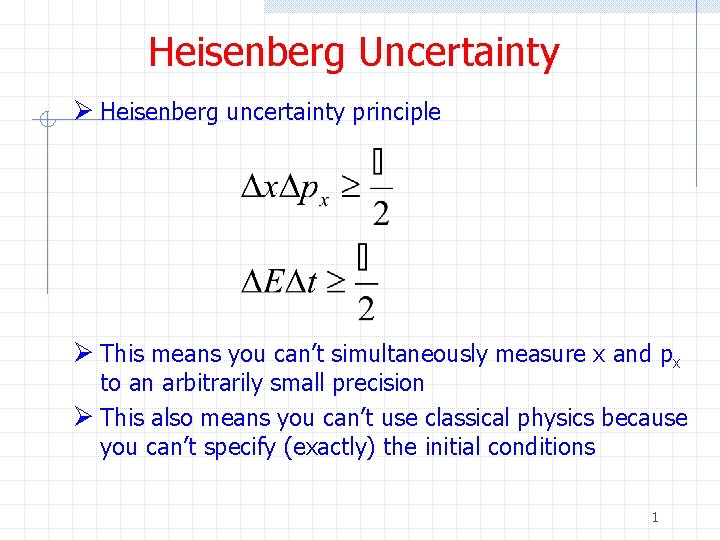 Source: slidetodoc.com
Source: slidetodoc.com
Lets discuss where this principle comes from and why its. There is another form of Heisenbergs uncertainty principle for simultaneous measurements of energy and timeIn equation form Delta EDelta tge cfrach4pi where Delta E is the uncertainty in energy and Delta t is the uncertainty in timeThis means that within a time interval Delta t it is not possible to. Very roughly it states that if we know everything about where a particle is located the uncertainty of position is small we know nothing about its momentum the uncertainty of momentum is large and vice versa. Chemistry Quantum Mechanical Model of the Atom Heisenberg Uncertainty Principle. Its submitted by admin in the best field.
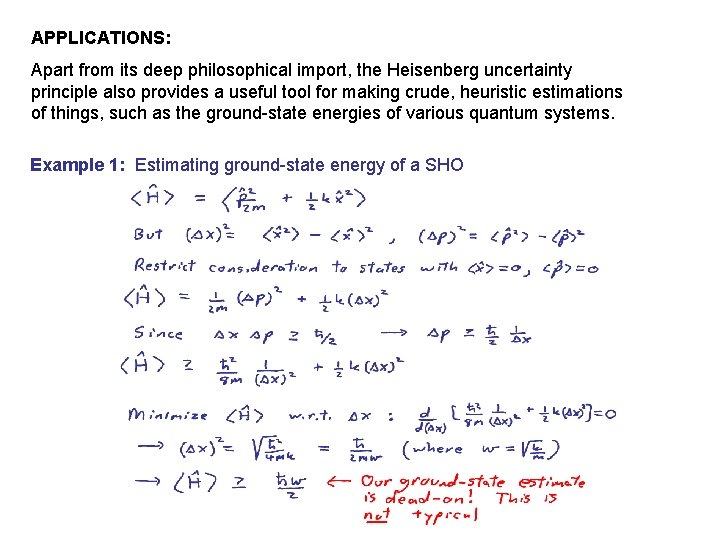 Source: slidetodoc.com
Source: slidetodoc.com
Heisenberg Uncertainty Principle Problems. Heisenbergs uncertainty principle is a key principle in quantum mechanics. Of a microparticle coordinate and the projection of its impulse along the coordinate axis as well as the uncertainty relation of microparticle time and energy falls into the ranks of the most important quantum relations that express the known uncertainty principle in a mathematical form. Learn more about Heisenberg Uncertainty Principle its examples formulas and equations and more here. 1 Δq x Δv ħm.

Its submitted by admin in the best field. The Heisenberg Uncertainty Principle is one example of our inability to have all possible information on a system. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. Solved Examples for Heisenberg Uncertainty Formula. We identified it from obedient source.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Its submitted by admin in the best field. This article will discuss the uncertainty principle as well Uncertainty Principle formula with examples. Here are a number of highest rated Heisenberg Uncertainty Principle Formula pictures on internet. 1 Δq x Δv ħm. Versions of the uncertainty principle also exist for other quantities as well such as.

This principle was given in 1927 by the German physicist Werner Heisenberg. 1 An electron in a molecule travels at a speed of 40ms. Electron spins around the orbital near speed of light. The uncertainty in the momentum Δp of the electron is 10 6 of its momentum. The most fundamental of these principles is the Heisenberg Uncertainty Principle HUP which states that in a quantum system only one property of a pair of conjugate properties can be known with certainty.
 Source: studylib.net
Source: studylib.net
Let us begin it. Its submitted by admin in the best field. Here are a number of highest rated Heisenberg Uncertainty Principle Formula pictures on internet. Δp is the uncertainty in momentum. 1 Δq x Δv ħm.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title heisenberg uncertainty principle example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






