Your Nonpolar covalent bond examples list images are ready. Nonpolar covalent bond examples list are a topic that is being searched for and liked by netizens now. You can Download the Nonpolar covalent bond examples list files here. Download all free vectors.
If you’re searching for nonpolar covalent bond examples list images information related to the nonpolar covalent bond examples list topic, you have come to the ideal site. Our website always gives you suggestions for seeking the maximum quality video and picture content, please kindly surf and find more informative video articles and images that fit your interests.
Nonpolar Covalent Bond Examples List. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also equally share the electrons. If the difference in electronegativity between two atoms is 04 or less the bond formed between the two atoms is a nonpolar covalent bond. Nonpolar Covalent Bonds for example can be found in gas molecules such as hydrogen gas nitrogen gas and so on. A bond between 2 nonmetal atoms that have the same electronegativity and therefore have equal sharing of the bonding electron pair.
 Polar Vs Nonpolar From users.stlcc.edu
Polar Vs Nonpolar From users.stlcc.edu
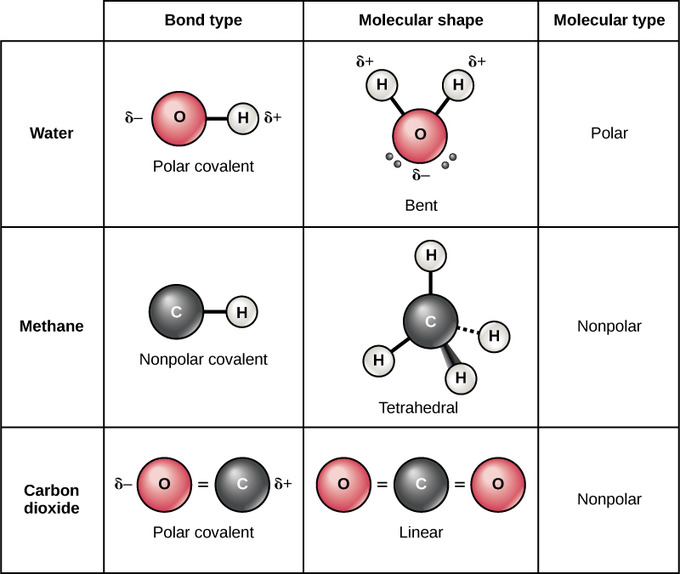
Here is a table listing molecules with polar and non. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms. Useful List of Molecules Non-Polar from READE. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. Answer 1 of 2. Nonpolar Covalent Bond Definition.
Examples of homonuclear nonpolar molecules are oxygen O 2 nitrogen N 2 and ozone O 3.
Carbon monoxide is a linear molecule but the. A covalent bond that has an equal sharing of electrons and the electronegativity difference is zero is called a nonpolar covalent bond. Example Nonpolar Covalent Bond is found in gas molecules like Hydrogen gas Nitrogen gas etc. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also uniformly share the electrons. In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar. The bonds between hydrogen and oxygen in water are an example of this type of bond.
 Source: bio.libretexts.org
Source: bio.libretexts.org
The more electrons they share the stronger the bond will be. Nonpolar covalent bond electronegativity scale. Here is a table listing molecules with polar and non. Carbon monoxide is a linear molecule but the. Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Example Nonpolar Covalent Bond is found in gas molecules like Hydrogen gas Nitrogen gas etc. Polar Bonds Polar bonds happen when two atoms form a molecule using a covalent bond. Nonpolar Covalent Bond Definition. For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. Examples of homonuclear nonpolar molecules are oxygen O 2 nitrogen N 2 and ozone O 3.
 Source: slidetodoc.com
Source: slidetodoc.com
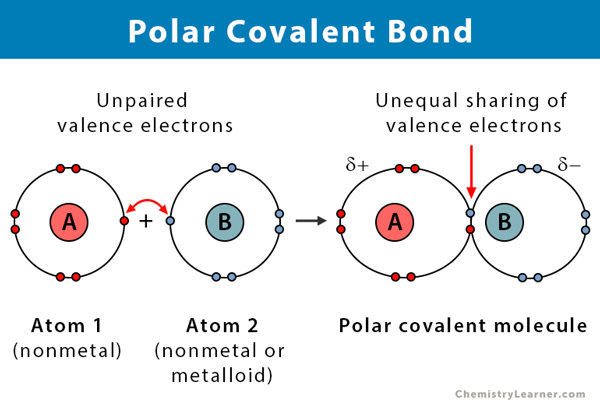
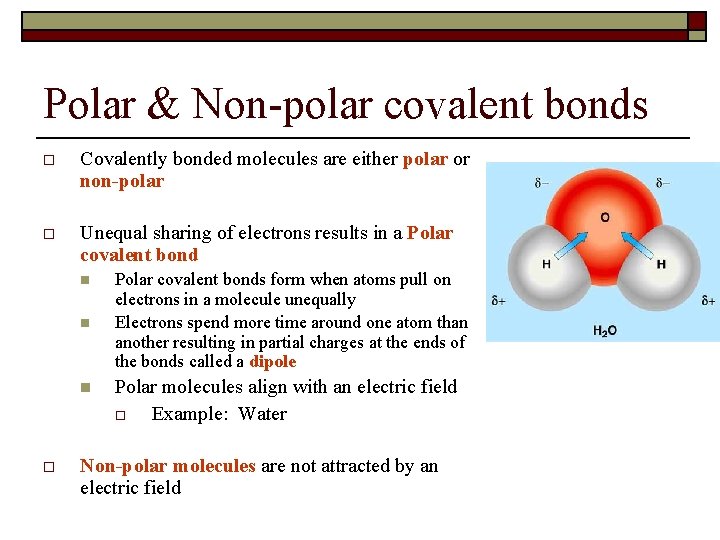
σ single covalent bond shared pair is between the tow atoms s orbital with s orbital. In more simple words a nonpolar covalent bond is formed when atoms share an equal number of electrons between them. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. Nitrous oxide nitric oxide ammonia. σ single covalent bond shared pair is between the tow atoms s orbital with s orbital.
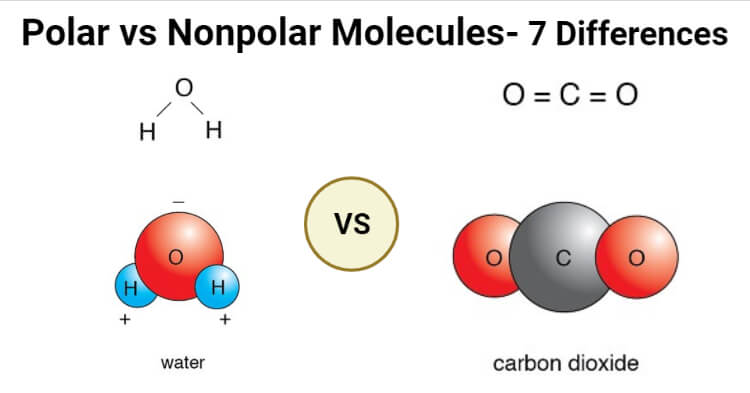 Source: thechemistrynotes.com
Source: thechemistrynotes.com
For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. A non-polar molecule is one that the electrons are distributed more symmetrically and thus does not have an abundance of charges at the opposite sides. When the two atoms share electrons there is also a change of electron density. Example Nonpolar Covalent Bond is found in gas molecules like Hydrogen gas Nitrogen gas etc. Nonpolar covalent41-17 electronegativity difference.
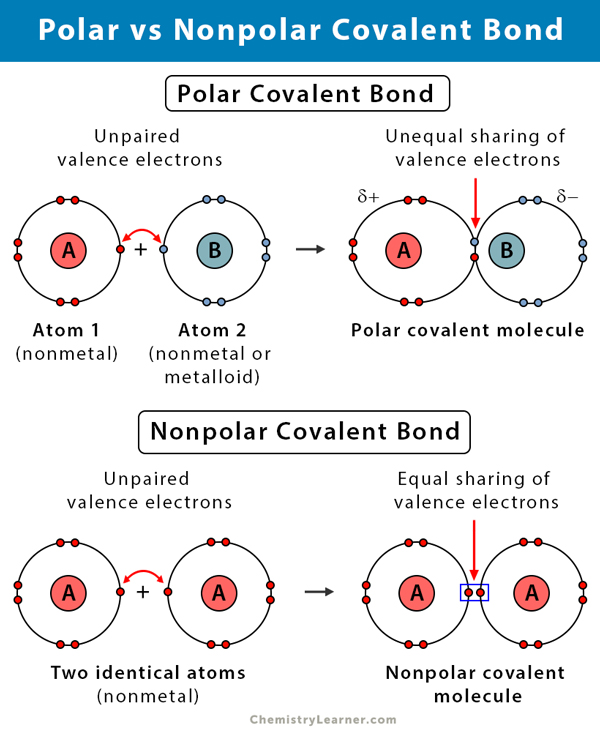 Source: chemistrylearner.com
Source: chemistrylearner.com
Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also uniformly share the electrons. Types of Covalent Bonds. A non-polar molecule is one that the electrons are distributed more symmetrically and thus does not have an abundance of charges at the opposite sides. Useful List of Molecules Non-Polar from READE. Other nonpolar molecules include carbon dioxide CO 2 and the organic molecules methane CH 4 toluene and gasoline.
 Source: slideplayer.com
Source: slideplayer.com
Nonpolar Covalent Bond Definition. For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. A bond between 2 nonmetal atoms that have the same electronegativity and therefore have equal sharing of the bonding electron pair.
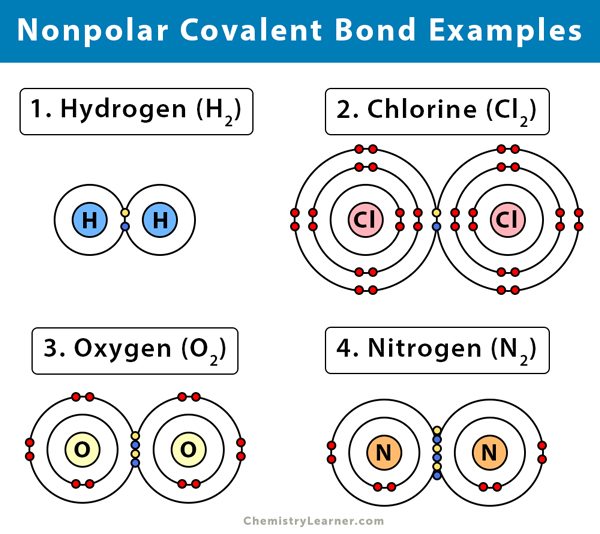 Source: chemistrylearner.com
Source: chemistrylearner.com
Over 17 electronegativity difference. In a molecule when electronegativity difference is less than 04 are referred as nonpolar covalent bonds. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also uniformly share the electrons. In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar. Example Nonpolar Covalent Bond is found in gas molecules like Hydrogen gas Nitrogen gas etc.
 Source: slideplayer.com
Source: slideplayer.com
Nitrous oxide nitric oxide ammonia. In a nonpolar covalent bond electrons are shared equally between atoms. An example of a nonpolar covalent bond is the bond between two hydrogen atoms because they uniformly share the electrons. If the difference in electronegativity between two atoms is 04 or less the bond formed between the two atoms is a nonpolar covalent bond. Here is a table listing molecules with polar and non.
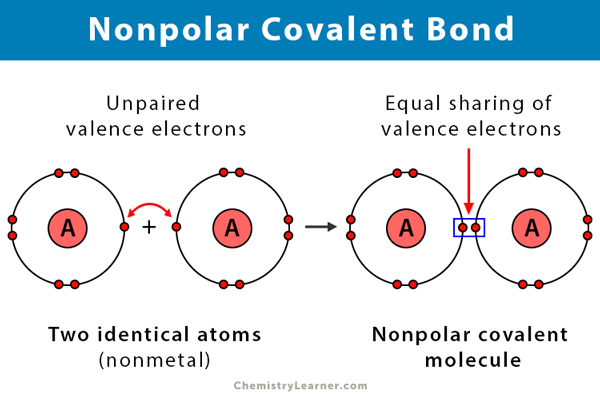 Source: chemistrylearner.com
Source: chemistrylearner.com
Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms. The more electrons they share the stronger the bond will be. In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar. The difference in electronegativity between two atoms is zero. Example Nonpolar Covalent Bond is found in gas molecules like Hydrogen gas Nitrogen gas etc.
 Source: quora.com
Source: quora.com
In a nonpolar covalent bond electrons are shared equally between atoms. It occurs whenever the atoms combining have a similar electron affinity. CO_2 O_2 CH_3 DNA non-polar amino acids Covalent bonds are common in the molecules of living organisms. The bonds between hydrogen and oxygen in water are an example of this type of bond. Nitrous oxide nitric oxide ammonia.
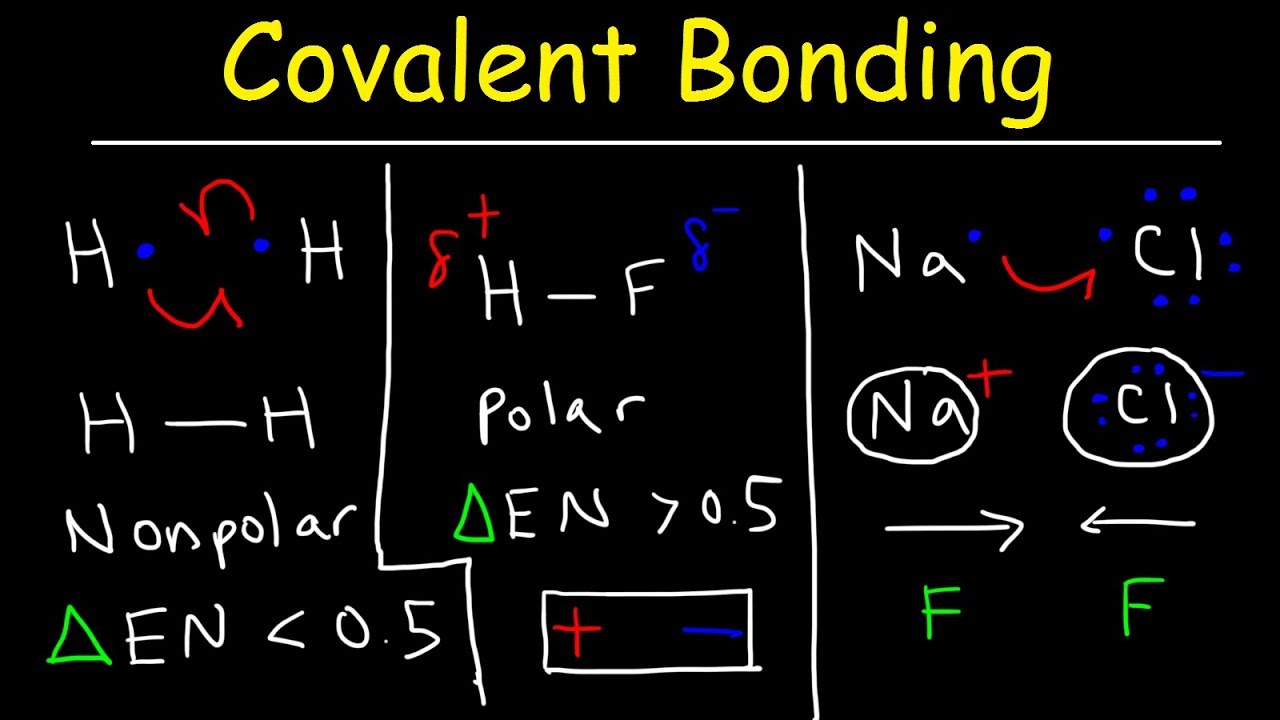 Source: youtube.com
Source: youtube.com
CO_2 O_2 CH_3 DNA non-polar amino acids Covalent bonds are common in the molecules of living organisms. Answer 1 of 2. The charges all cancel out each other. Nonpolar covalent bonds are very strong and they require a huge amount of energy to break the bond. Polar covalent bond Hydrogen chloride also called hydrochloric acid is.
 Source: study.com
Source: study.com
Carbon monoxide is a linear molecule but the. In a molecule when electronegativity difference is less than 04 are referred as nonpolar covalent bonds. In polar covalent bonds unequal attraction of electrons is seen. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds.
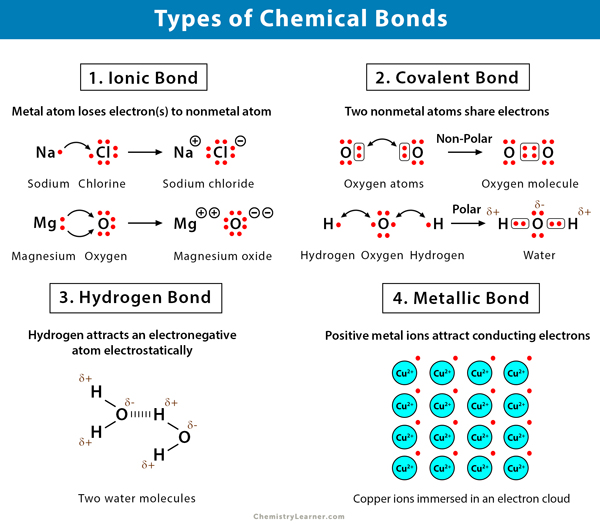 Source: chemistrylearner.com
Source: chemistrylearner.com
In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar. A covalent bond that has an equal sharing of electrons and the electronegativity difference is zero is called a nonpolar covalent bond. The charges all cancel out each other. For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. Non-polar covalent bonds appear between two atoms of the same element or between different elements that equally share.
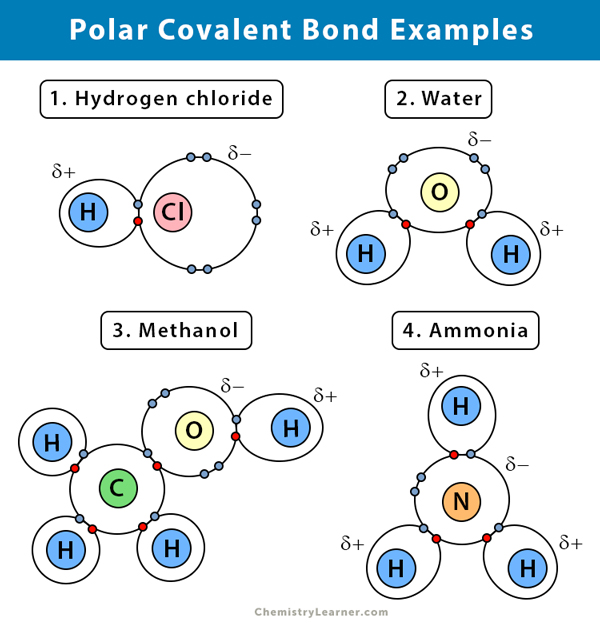 Source: chemistrylearner.com
Source: chemistrylearner.com
In polar covalent bonds unequal attraction of electrons is seen. Polar covalent bond Hydrogen chloride also called hydrochloric acid is. Examples of nonpolar bond. Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na and negative charged Cl- ions. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms.
 Source: users.stlcc.edu
Source: users.stlcc.edu
If the electrons are not shared equally then there will be a. Polar Bonds Polar bonds happen when two atoms form a molecule using a covalent bond. It occurs whenever the atoms combining have a similar electron affinity. A notable exception is carbon monoxide CO. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond.
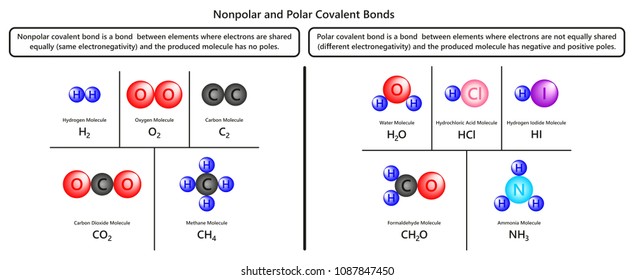 Source: shutterstock.com
Source: shutterstock.com
When the two atoms share electrons there is also a change of electron density. CO_2 O_2 CH_3 DNA non-polar amino acids Covalent bonds are common in the molecules of living organisms. This type of covalent bond is formed when two atoms share an equal number of electrons. Nonpolar covalent bonds are very powerful bonds demanding a large amount of energy to break. A non-polar molecule is one that the electrons are distributed more symmetrically and thus does not have an abundance of charges at the opposite sides.
 Source: study.com
Source: study.com
σ single covalent bond shared pair is between the tow atoms s orbital with s orbital. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms. Examples of nonpolar bond. Polar covalent bond Hydrogen chloride also called hydrochloric acid is. If the electrons are not shared equally then there will be a.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
This bonds are created is by sharing electrons. CO_2 O_2 CH_3 DNA non-polar amino acids Covalent bonds are common in the molecules of living organisms. It occurs whenever the atoms combining have a similar electron affinity. When the two atoms share electrons there is also a change of electron density. Nonpolar covalent bonds are very strong and they require a huge amount of energy to break the bond.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nonpolar covalent bond examples list by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.