Your Polar covalent bond examples list images are available. Polar covalent bond examples list are a topic that is being searched for and liked by netizens today. You can Find and Download the Polar covalent bond examples list files here. Get all free vectors.
If you’re searching for polar covalent bond examples list pictures information related to the polar covalent bond examples list interest, you have visit the ideal blog. Our site always provides you with hints for seeking the highest quality video and picture content, please kindly hunt and locate more informative video content and graphics that fit your interests.
Polar Covalent Bond Examples List. The orientation of the various polar bonds in these molecules is such that their polarities cancel each other. Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms. The electronegativity value of oxygen is 344 while the electronegativity of hydrogen is 220. Helium He Bromine Br 2.
 Lesson 37 Covalent Compounds Objectives The Student Will Define Covalent Bond And Molecular Compound The Student Will Classify Bonds As Ionic Polar Ppt Download From slideplayer.com
Lesson 37 Covalent Compounds Objectives The Student Will Define Covalent Bond And Molecular Compound The Student Will Classify Bonds As Ionic Polar Ppt Download From slideplayer.com
The orientation of the various polar bonds in these molecules is such that their polarities cancel each other. Below is a list of such molecules with their formulae. Specifically when the difference in electronegativities of the two atoms in the bond is between 04 and 17. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. In general the electronegativity difference must be 05 or more before the bond is labeled as a polar covalent bond instead of nonpolar covalent bond. Such types of covalent bonds are Polar Covalent bonds.
The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220.
Below is a list of such molecules with their formulae. Examples of Nonpolar Covalent Molecules with Polar Covalent Bonds. The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible. If the difference is less than 05 the atoms normally form a non-polar covalent bond while ionic bonds are formed when the difference is greater than 20. Polar Molecules. Here is a table listing molecules with polar and non.
 Source: slideplayer.com
Source: slideplayer.com
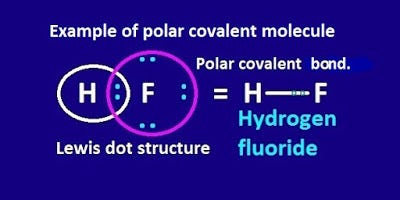
Examples of Molecules with Polar Covalent Bonds. Polar Covalent Bond Examples of Molecules with Polar Covalent Bond. Another example of a polar covalent bond is between a hydrogen and a chlorine atom. Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na and negative charged Cl- ions. A covalent bond in which the electron density is unevenly shared between the two bonded atoms due to a difference in electronegativity or due to inductive effects.
 Source: slideplayer.com
Source: slideplayer.com
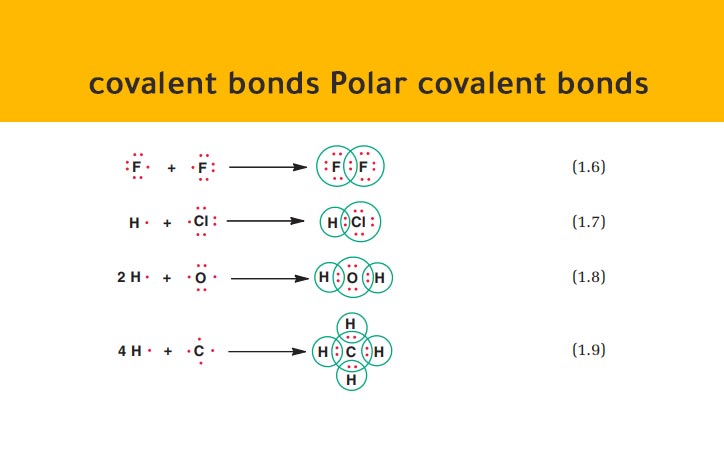
In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar. Specifically when the difference in electronegativities of the two atoms in the bond is between 04 and 17. The oxygen side of the molecule has a net negative charge while the two. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. This happens when there is a difference between the electronegativity values of each atom.
 Source: kgghosh1990.medium.com
Source: kgghosh1990.medium.com
What Is Polar Covalent Bond. An absolute difference forms an ionic bond while a minor difference forms a polar covalent bond. The bonds between hydrogen and oxygen in water are an example of this type of bond. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. Water consists of a covalent bond containing hydrogen and oxygen bonding together to make H 2 O.
 Source: chemistrylearner.com
Source: chemistrylearner.com
What Is Polar Covalent Bond. The inequality in electron distribution accounts for the best shape of the molecule. Water H2O is a molecule having a polar covalent bond. In this atomic molecule two hydrogen atoms share their single electrons with the oxygen atom which shares its own two electrons in return. In polar covalent bonds the electrons are.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Types of Covalent Bonds. Polar Molecules. Examples of Covalent Bond. Answer 1 of 3. This happens when there is a difference between the electronegativity values of each atom.
 Source: chemistrylearner.com
Source: chemistrylearner.com
A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. The electronegativity value of oxygen is 344 while the electronegativity of hydrogen is 220. Such types of covalent bonds are Polar Covalent bonds. This happens when there is a difference between the electronegativity values of each atom. An example is water.

Below is a list of such molecules with their formulae. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible. The degree of polarity of a bond particularly depends on the difference in electronegativities of the two atoms bonded together and partly on other factors such as the size of the atoms. What Is Polar Covalent Bond.
 Source: chemistrylearner.com
Source: chemistrylearner.com
An absolute difference forms an ionic bond while a minor difference forms a polar covalent bond. This happens when there is a difference between the electronegativity values of each atom. Polar Molecules. An extreme difference forms an. The degree of polarity of a bond particularly depends on the difference in electronegativities of the two atoms bonded together and partly on other factors such as the size of the atoms.
 Source: researchgate.net
Source: researchgate.net
Water H2O is a polar bonded molecule. The inequality in electron distribution accounts for the bent shape of the molecule. Examples of Molecules with Polar Covalent Bonds. The terms polar bond and polar covalent bond are generally used interchangeably. The electronegativity amount of oxygen is 344 while the electronegativity of hydrogen is 220.
 Source: shutterstock.com
Source: shutterstock.com
Nonpolar Covalent Bond Examples. Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms. What Is Polar Covalent Bond. An extreme difference forms an. It is said that water is the universal solvent but this does not mean that it dissolves universally but rather that due to its abundance it is a suitable solvent to dissolve polar substances Helmenstine 2017.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Nonpolar Covalent Bond Examples. If the electronegativity variation between the two atoms is between 05 and 20 the atoms form a polar covalent bond. Examples of Covalent Bond. Helium He Bromine Br 2. A polar covalent bond occurs when atoms are shared unequally in a covalent bond.
 Source: chemistry1science.com
Source: chemistry1science.com
Because of this. The oxygen side of the molecule features. An extreme difference forms an. The bonds between hydrogen and oxygen in water are an example of this type of bond. Water H 2 O is a polar bonded molecule.

An example is water. In general the electronegativity difference must be 05 or more before the bond is labeled as a polar covalent bond instead of nonpolar covalent bond. If the electronegativity variation between the two atoms is between 05 and 20 the atoms form a polar covalent bond. A bond between 2 nonmetal atoms that have the same electronegativity and therefore have equal sharing of the bonding electron pair. Polar Covalent Bond Examples of Molecules with Polar Covalent Bond.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Water H2O is a polar bonded molecule. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. In general the electronegativity difference must be 05 or more before the bond is labeled as a polar covalent bond instead of nonpolar covalent bond. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. Luckily you can look up electronegativity on a table to foretell whether or not atoms are likely to form polar covalent bonds.
 Source: chemistrytalk.org
Source: chemistrytalk.org
Luckily you can look up electronegativity on a table to foretell whether or not atoms are likely to form polar covalent bonds. A covalent bond in which the electron density is unevenly shared between the two bonded atoms due to a difference in electronegativity or due to inductive effects. In this bond the chlorine atom spends more time with the electrons than the hydrogen atom. This type of bond can only occur when the difference in electronegativity is between 04 and 20. The degree of polarity of a bond particularly depends on the difference in electronegativities of the two atoms bonded together and partly on other factors such as the size of the atoms.
 Source: biologyfisiology.blogspot.com
Source: biologyfisiology.blogspot.com
The electronegativity value of oxygen is 344 while the electronegativity of hydrogen is 220. Thus in an atom the number of electrons shared by the adjacent atoms will be the same. The water H 2 O is the most classic example of a polar molecule. The degree of polarity of a bond particularly depends on the difference in electronegativities of the two atoms bonded together and partly on other factors such as the size of the atoms. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The electronegativity amount of oxygen is 344 while the electronegativity of hydrogen is 220. Polar Covalent Bond Examples of Molecules with Polar Covalent Bond. Another example of a polar covalent bond is between a hydrogen and a chlorine atom. An example is water. Water H2O is a polar bonded molecule.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
Polar Covalent Bond Examples of Molecules with Polar Covalent Bond. An absolute difference forms an ionic bond while a minor difference forms a polar covalent bond. A covalent bond in which the electron density is unevenly shared between the two bonded atoms due to a difference in electronegativity or due to inductive effects. The inequality in electron distribution accounts for the best shape of the molecule. The terms polar bond and polar covalent bond are generally used interchangeably.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title polar covalent bond examples list by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






