Your Zeroth law of thermodynamics examples images are ready. Zeroth law of thermodynamics examples are a topic that is being searched for and liked by netizens now. You can Download the Zeroth law of thermodynamics examples files here. Download all free photos and vectors.
If you’re searching for zeroth law of thermodynamics examples images information related to the zeroth law of thermodynamics examples interest, you have come to the right site. Our website always provides you with hints for seeking the maximum quality video and image content, please kindly hunt and locate more informative video articles and graphics that fit your interests.
Zeroth Law Of Thermodynamics Examples. 4 Cold water and hot water. The total energy of an isolated system is neither created nor destroyed the amount of energy remains constant Energy is transformed from one form to another. The zeroth law of thermodynamics states that if two systems that are in thermal equilibrium with a third system they are also in equilibrium with each other It is also known as the zeroth principle of thermodynamics. 5 Vegetables in your refrigerator.
 The Zeroth Law Of Thermodynamics Ppt Download From slideplayer.com
The Zeroth Law Of Thermodynamics Ppt Download From slideplayer.com
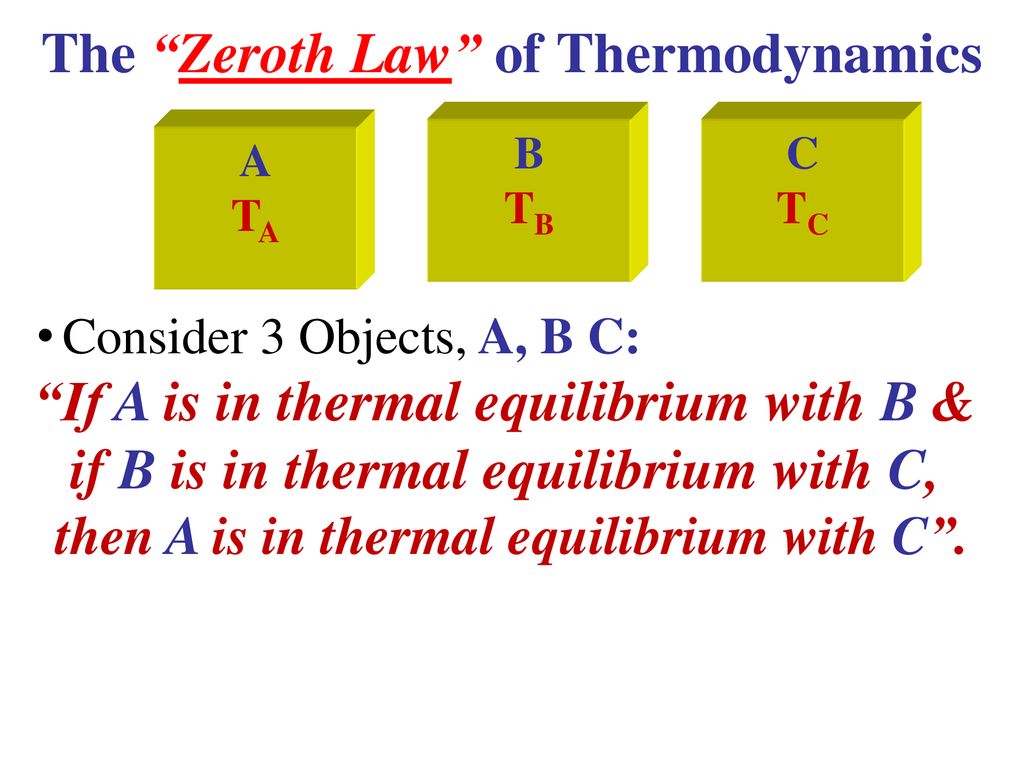
If we want to accurately measure temperature. The Zeroth Law of Thermodynamics The zeroth law is incredibly important as it allows us to define the concept of a temperature scale. The total energy of an isolated system is neither created nor destroyed the amount of energy remains constant Energy is transformed from one form to another. If A is in equilibrium with B and A is also in thermal stability with a third body C we can conclude that B is in thermal. Thermodynamics is the study of the relationship between heat temperature work and energy. Applicationsexamples of Zeroth law of thermodynamics.
Hence the Zeroth law of thermodynamics is stated as If two systems are each in thermal equilibrium with third system they are also in thermal equilibrium with each other.
6 Thermometer for measuring body temperature. The total energy of an isolated system is neither created nor destroyed the amount of energy remains constant Energy is transformed from one form to another. But this is not easy to keep name of zeroth law. Answer 1 of 2. The law is crucial for the mathematical formulation of thermodynamics or to put it another way for expressing the mathematical definition of temperature. If we want to accurately measure temperature.
 Source: slidetodoc.com
Source: slidetodoc.com
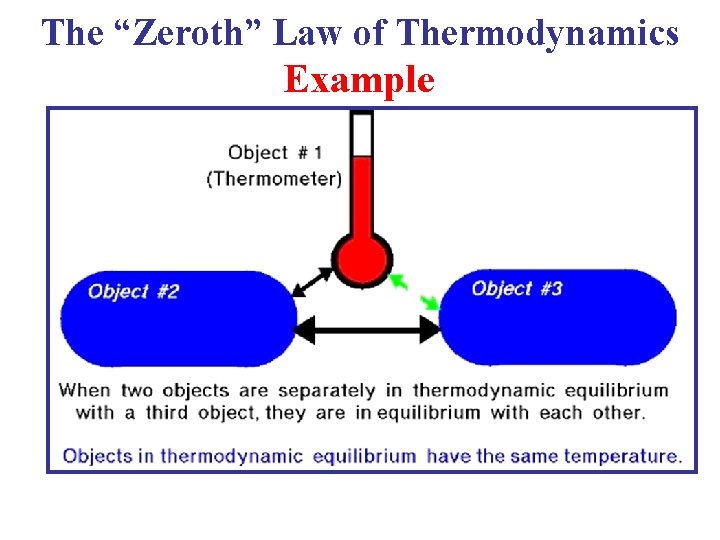
7 Temperature measurement in refrigerator. 7 Temperature measurement in refrigerator. But our point is not that to know the discovery of zeroth law. The law of thermal equilibrium the zeroth law is an important law in thermodynamics. But dey cudnt change d name of 1st law as it was.
 Source: study.com
Source: study.com
Zeroth law is so called because before zeroth law 1st law was made. Based on the zeroth law of thermodynamics the sugar and the milk are in equilibrium. To prepare coffee three ingredients are combined in a mug. But our point is not that to know the discovery of zeroth law. The Zeroth Law of Thermodynamics states that systems in thermal equilibrium are at the same temperature.
 Source: lawofthermodynamicsinfo.com
Source: lawofthermodynamicsinfo.com
3 A thermostat in your room. 3 A thermostat in your room. The Zeroth Law of Thermodynamics states that systems in thermal equilibrium are at the same temperature. 5 Vegetables in your refrigerator. Consider the following examples.
 Source: student-baba.com
Source: student-baba.com
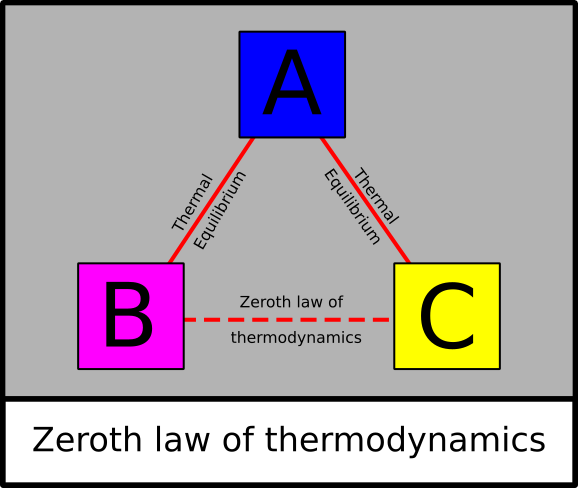
Based on the zeroth law of thermodynamics the sugar and the milk are in equilibrium. The zeroth law of thermodynamics states that if two systems that are in thermal equilibrium with a third system they are also in equilibrium with each other It is also known as the zeroth principle of thermodynamics. Zeroth law is so called because before zeroth law 1st law was made. The Zeroth Law of Thermodynamics The zeroth law is incredibly important as it allows us to define the concept of a temperature scale. If two bodies are in thermal equilibrium with a third body they are in thermal equilibrium with each other as well.

There are total four laws of thermodynamics. The zeroth law of thermodynamics states that if two systems that are in thermal equilibrium with a third system they are also in equilibrium with each other It is also known as the zeroth principle of thermodynamics. Examples of how to use zeroth law of thermodynamics in a sentence from the Cambridge Dictionary Labs. For example install a sheet of copper and use silicone caulking to hold the copper sheet in place so that you have two separate sides of. The Zeroth Law of Thermodynamics states that systems in thermal equilibrium are at the same temperature.

5 Vegetables in your refrigerator. 2 Hot coffee becomes cold after some time. If two bodies are in thermal equilibrium with a third body they are in thermal equilibrium with each other as well. It is fourth law of thermodynamics but know as zeroth law. Build a tank which can hold water and put a diathermic wall between the two sides of the tank.
 Source: slideplayer.com
Source: slideplayer.com
First Law of Thermodynamics First law of thermodynamics also known as the law of conservation of energy states that energy can neither be created nor destroyed but it can be changed from one form to another. Suppose if someone makes a ham and cheese sandwich and. 1 2 3 A more fundamental statement was later labelled as the zeroth law after the first three laws had been established. Some daily living examples that exemplify the zeroth law of thermodynamics are. If two bodies are in thermal equilibrium with a third body they are in thermal equilibrium with each other as well.
 Source: slideplayer.com
Source: slideplayer.com
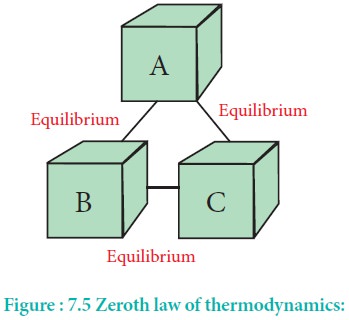
The law is crucial for the mathematical formulation of thermodynamics or to put it another way for expressing the mathematical definition of temperature. Examples of how to use zeroth law of thermodynamics in a sentence from the Cambridge Dictionary Labs. By both numerical simulations and by using exact expressions for free energy and microcanonical entropy it is shown that if two systems with the same intensive parameters but with negative specific heat are thermally coupled they undergo a process in which the total entropy. But this is not easy to keep name of zeroth law. Despite such importance this law was not fully realized for soo long until 1935 when.
 Source: brainkart.com
Source: brainkart.com
We show that systems with negative specific heat can violate the zeroth law of thermodynamics. When you go to doctor for complaining of fever then he checks our body temperature using thermometer. The law is crucial for the mathematical formulation of thermodynamics or to put it another way for expressing the mathematical definition of temperature. But this is not easy to keep name of zeroth law. Quantum mechanics in the limit 0but.
 Source: reelatscience.blogspot.com
Source: reelatscience.blogspot.com
4 Cold water and hot water. If two systems are each in thermal equilibrium with a third they are also in thermal. First Law of Thermodynamics First law of thermodynamics also known as the law of conservation of energy states that energy can neither be created nor destroyed but it can be changed from one form to another. Build a tank which can hold water and put a diathermic wall between the two sides of the tank. Zeroth law is so called because before zeroth law 1st law was made.
 Source: geeksforgeeks.org
Source: geeksforgeeks.org
Hence the Zeroth law of thermodynamics is stated as If two systems are each in thermal equilibrium with third system they are also in thermal equilibrium with each other. Thermodynamics is the study of the relationship between heat temperature work and energy. But this is not easy to keep name of zeroth law. For example install a sheet of copper and use silicone caulking to hold the copper sheet in place so that you have two separate sides of. It is fourth law of thermodynamics but know as zeroth law.
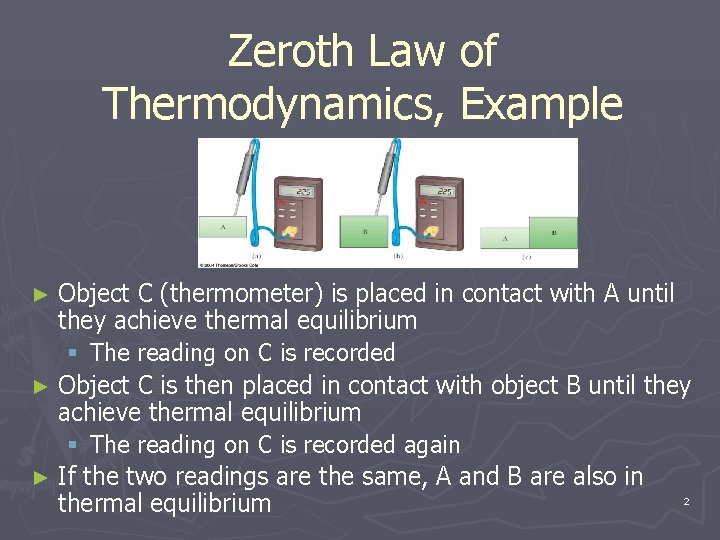 Source: slidetodoc.com
Source: slidetodoc.com
Build a tank which can hold water and put a diathermic wall between the two sides of the tank. If A is in equilibrium with B and A is also in thermal stability with a third body C we can conclude that B is in thermal. 4 Cold water and hot water. The Zeroth law is incredibly important as it allows us to define the concept of a temperature scale. Build a tank which can hold water and put a diathermic wall between the two sides of the tank.
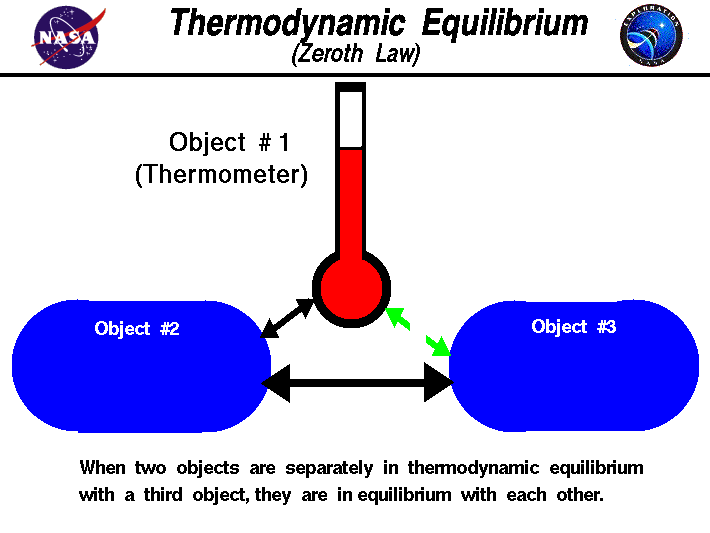 Source: grc.nasa.gov
Source: grc.nasa.gov
Zeroth Law of Thermodynamics Example and Applications. The law of thermal equilibrium the zeroth law is an important law in thermodynamics. Although the definition seems very technical and challenging to understand numerous everyday examples apply this thermodynamic principle. The Zeroth Law of Thermodynamics states that systems in thermal equilibrium are at the same temperature. 3 A thermostat in your room.
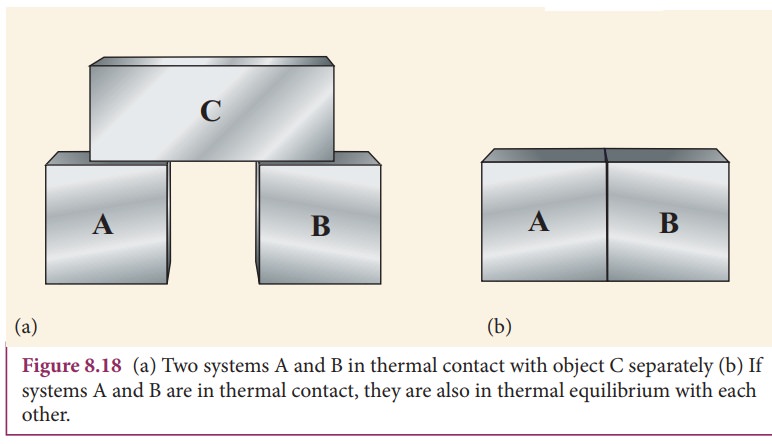 Source: brainkart.com
Source: brainkart.com
The Zeroth Law of Thermodynamics The zeroth law is incredibly important as it allows us to define the concept of a temperature scale. We show that systems with negative specific heat can violate the zeroth law of thermodynamics. When you go to doctor for complaining of fever then he checks our body temperature using thermometer. The most common use of this concept is to compare the temperatures of different things. 3 A thermostat in your room.
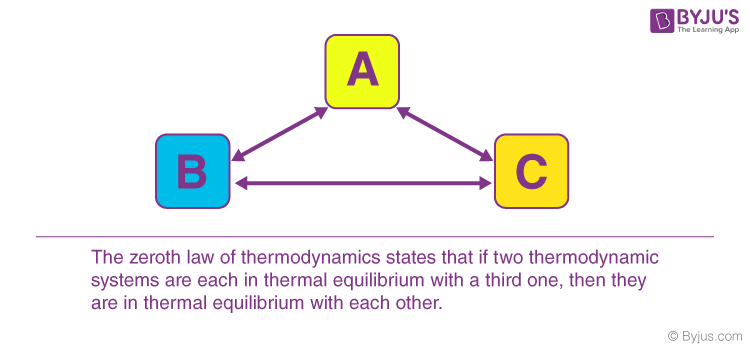 Source: byjus.com
Source: byjus.com
65 C coffee 65 C milk and 65 C sugar which is in thermal equilibrium with the coffee. Some daily living examples that exemplify the zeroth law of thermodynamics are. Answer 1 of 2. Based on the zeroth law of thermodynamics the sugar and the milk are in equilibrium. The zeroth law of thermodynamics is one of the four laws of thermodynamics which states that if two systems are in thermal equilibrium with a third system then they are in thermal equilibrium with one another.
 Source: study.com
Source: study.com
We show that systems with negative specific heat can violate the zeroth law of thermodynamics. Applicationsexamples of Zeroth law of thermodynamics. Zeroth Law of Thermodynamics Examples. Thermodynamics is the study of the relationship between heat temperature work and energy. Based on the zeroth law of thermodynamics the sugar and the milk are in equilibrium.
 Source: unacademy.com
Source: unacademy.com
1 2 3 A more fundamental statement was later labelled as the zeroth law after the first three laws had been established. Suppose if someone makes a ham and cheese sandwich and. The zeroth law of thermodynamics states that if two systems that are in thermal equilibrium with a third system they are also in equilibrium with each other It is also known as the zeroth principle of thermodynamics. Hence the Zeroth law of thermodynamics is stated as If two systems are each in thermal equilibrium with third system they are also in thermal equilibrium with each other. 3 A thermostat in your room.
 Source: youtube.com
Source: youtube.com
It is fourth law of thermodynamics but know as zeroth law. Zeroth Law of Thermodynamics Examples. Applicationsexamples of Zeroth law of thermodynamics. The zeroth law of thermodynamics states that. If A is in equilibrium with B and A is also in thermal stability with a third body C we can conclude that B is in thermal.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title zeroth law of thermodynamics examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






